RESEARCH ARTICLE
Effect of IV Midazolam Premedication on the Recovery of Pediatric Patients after Isoflurane Anesthesia for Cochlear Implant Surgery
Mona R. Hosny1, Ashraf N. Saleh1, *, Wail A. Abdelaal1, Tahany M. Rabie2
Article Information
Identifiers and Pagination:
Year: 2019Volume: 13
First Page: 47
Last Page: 52
Publisher ID: TOATJ-13-47
DOI: 10.2174/2589645801913010047
Article History:
Received Date: 18/03/2019Revision Received Date: 26/05/2019
Acceptance Date: 24/06/2019
Electronic publication date: 24/07/2019
Collection year: 2019

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose:
Midazolam, given by varying routes, is widely used as a premedication. This study was performed to investigate the effect of IV midazolam premedication on the recovery characteristics of isoflurane anesthesia in pediatric patients undergoing cochlear implant surgery.
Methods:
In this double-blind randomized study, a total of 60 unilateral cochlear implants procedures were performed on 60 children aged 1 – 6 years. They were 29 males (48.3%) and 31 females (51.7%). Patients were randomly allocated in one of the two groups (M and S). Each group included 30 participants. Patients in group M received 0.01 mg/kg IV midazolam in 2 ml of 0.9% saline, while patients in group S received an equal volume of 0.9% saline, two minutes before induction. Recovery times from the discontinuation of isoflurane were recorded. Postoperative pain was assessed using the Objective Pain Discomfort Score (OPDS). Emergence Agitation (EA) was recorded based on Aono’s four-point scale.
Results:
There were statistically significant differences between patients pre-medicated with IV midazolam and those of the normal saline group in all the measured recovery parameters (p<0.001). Patients in group M scored higher than those in Group S on the OPDS. Yet, this difference didn't show any statistical significance (p=0.438) Among patients pre-medicated with midazolam, 17 (56.6%) suffered from EA compared to 12 (40%) patients from the other group. This difference did not reach statistical significance (p=0.196).
Conclusion:
Premedication with IV midazolam delayed recovery in pediatric patients undergoing moderately-long procedures when isoflurane was used as the inhalation anesthetic, while its effect on EA remained uncertain.
1. INTRODUCTION AND BACKGROUND
The majority of pediatric patients suffer from preoperative anxiety [1] that is commonly treated with behavioral methods such as parental presence during the induction of anesthesia or with pharmacological interventions such as sedative premedication [2]. The goal is to treat amnesia, by optimizing preoperative conditions, preventing psychological trauma, and most importantly, by anxiolysis [3].
Midazolam, a benzodiazepine derivative, can be employed by multiple routes [oral, intramuscular and Intravenous (IV)] to adults and children [4]. Its elimination half-life of 1.20 hours makes it particularly suitable for brief procedures [5]. In children, premedication with oral midazolam seems to be a satisfactory anxiolytic before anesthesia [6, 7], reducing the psychological impact of hospitalization after surgery [8]. However, its routine use before anesthetic induction is controversial as the specific effect of midazolam in blocking explicit memory while preserving implicit memory is a serious problem especially in children [9, 10].
In the same context, the literature shows contradicting results regarding the effect of midazolam premedication on recovery from anesthesia [1, 2, 11, 12]. Several studies have reported no effect on recovery characteristics after midazolam premedication when using inhaled anesthesia with halothane [6, 7, 12]. To the best of our knowledge, the effect of IV midazolam on recovery from the ambulatory isoflurane anesthesia is still unclear.
Cochlear implantation is a surgical therapeutic option for patients suffering from irreversible hearing loss and deaf-mutism [13]. Pediatric cochlear implantation is a specialized, complicated, costly and challenging procedure.
The procedure is performed via standard trans-mastoid posterior tympanotomy approach with the preservation of the facial nerve and the functional integrity of the cochlea which requires general anesthesia as well as safe hypotensive balanced anesthesia. The technique of anesthesia plays a very important role in the success of cochlear implant surgery in addition to producing conditions which facilitate the use of nerve stimulators and management of post-operative complications like nausea, vomiting and vertigo [14]. Moreover, the anesthesiologist may encounter difficulties in communication-impaired patients [13, 15].
2. METHODS
2.1. Study Design and Setting
This was a double-blind randomized study conducted at the Ain Shams University Hospitals (ASUHs) in the period from October 2018 to December 2018.
2.1.1. Study Population
The study enrolled 60 pediatric patients after informed consent was obtained from their parents or their legal guardians. The work was approved by the Research Ethics Committee of ASUHs (FMASU R51/ 2018) and in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments in humans.
The study was prospectively registered with the Pan African Clinical Trial Registry (PACTR) with Registration Number PACTR201810739290859 and conducted in the period from October 2018 to December 2018.
Eligibility criteria included children aged 1-6 years, American Society of Anesthesiologists (ASA) Physical Status (PS) Class I and II classification. Exclusion criteria included mentally disabled children, allergy to midazolam and patients on medication that could interact with or affect the pharmacokinetics and pharmacodynamics of midazolam such as clarithromycin, antiepileptics or sedatives.
2.1.2. Data Collection
Demographic characteristics and relevant clinical data were collected for each patient using a standardized data collection form.
2.1.3. Preoperative Preparation
Functional assessment of the hearing capability of all patients was made as well as radiological studies including CT, MRI of the temporal bone.
All the patients fasted 4-6 hours before surgery. One hour before presenting to the theatre, patients were pre-medicated with chloral hydrate syrup 30 mg/kg and topical anesthesia (EMLA cream, AstraZeneca, UK) was applied on both the hands. An IV cannula was then inserted in the pre-induction phase.
2.1.4. Patients’ Randomization and Interventions
Patients were randomly allocated in one of the two groups (M and S) by a computer-generated random numbers list and the use of opaque sealed envelopes. Each group included 30 participants. Patients in group M received 0.01 mg/kg of IV midazolam in 2 ml of 0.9% saline, while patients in group S received an equal volume of 0.9% saline, two minutes before induction and after parental separation. An anesthesiology technician/nurse prepared the IV solution to be administered, based on the patient’s assigned group, handed it over to the investigating anesthesiologist and played no role in the patient’s assessment. The patients, their parents, the investigating anesthesiologist, the surgeon, and the Post Anesthesia Care Unit (PACU) nurse remained blinded to the groups.
2.1.5. Intraoperative Management
The children were then transferred to the operating room where standard ASA monitors were placed before the induction of anesthesia, for recording heart rate, blood pressure and SpO2. Infusion of lactated Ringer’s solution containing 2.5% dextrose was started.
Anesthesia was induced with sevoflurane with a gradual increase every few breaths up to 8 vol % inspired concentration of an air oxygen mixture via face mask, and 0.4 mg/kg atracurium was used as a neuromuscular blocker just at induction. Anesthesia was maintained with a low flow oxygen air mixture and isoflurane 2 – 2.5% Minimum Alveolar Concentration (MAC) to maintain mean arterial blood pressure with 25% reduction in the initial reading to achieve optimum hypotensive anesthesia necessary for the surgery. Pressure controlled ventilation was used and normocapnia (ETCO2 35-45 mm Hg) was maintained. Analgesia was achieved with fentanyl 1 ug/kg for all the patients as a single dose after skin incision and 15 mg/kg of IV paracetamol was given one hour after skin incision with dexamethasone four mg, a semi-closed circle system was used throughout anesthesia.
Surgery was performed in a standard fashion. A postauricular skin flap was elevated and a complete mastoidectomy with posterior tympanotomy was performed followed by cochleostomy inferior and anterior to the round window membrane. The electrode array was inserted, and the wound was closed in layers. Electrical impedance and neural response telemetry were performed before extubation. A plain radiograph was obtained intraoperatively to confirm the placement of the electrode array.
At the end of the surgery, diclofenac sodium 12.5 mg suppositories were given for postoperative analgesia together with IV granisetron (20 µg/kg) as an antiemetic, all the anesthetics were discontinued, with the reversal of muscle relaxants and 100% oxygen was delivered. The oropharynx was suctioned and extubation was performed when spontaneous breathing was regarded as adequate and good tidal volumes were achieved. It is to be noted that all surgeries were performed by the same surgeon.
2.1.6. Postoperative Assessment
In PACU, vital signs (heart rate, blood pressure, SpO2) were monitored until the child was fully awake. Any adverse event (vomiting, airway difficulty such as laryngospasm, and airway edema) was recorded. The following recovery times from the discontinuation of isoflurane were recorded:
(1) Spontaneous eyes opening.
(2) Scoring full points on the modified Aldrete scores (score of 9 or more) [16].
(3) Interacting with the nurse or the parent.
(4) Drinking.
(5) Hospital discharge.
Postoperative pain was assessed using the Objective Pain Discomfort Score (OPDS). The score relies on assessing blood pressure, crying; movement; agitation; and verbal complaints of pain or body language. Each criterion is given a score of 0-2, with a maximum possible OPDS score of 10 [17, 18].
Emergence Agitation (EA) was recorded based on Aono’s Four-Point Scale (AFPS); in which, 1 = calm and quiet, 2 = not calm but consolable, 3 = agitated, restless and not consolable, and 4 = excited or disoriented. EA was defined as an AFPS of ≥ 3 [19, 20].
2.2. Statistical Analysis and Sample Size Determination
It was predicted that in order to detect a 25% difference in discharge times, with a mean value of 80 minutes and a SD of 20 minutes, a minimum of 28 patients would be required in each group. In the current study, we included 30 patients. This gave the study a power of 80% at a significance level of 5%.
Results were statistically calculated with a statistical package for social science and were presented as mean + SD, 95% Confidence Intervals (CI) or number and percent. Tests used for analysis were Student’s t-test, Mann-Whitney U-test, Chi square or Fisher’s exact test as appropriate. A P-value < 0.05 was considered significant.
3. RESULTS
A total of 60 unilateral cochlear implants procedures were performed on 60 children aged 1-6 years. They were 29 males (48.3%) and 31 females (51.7%). No intraoperative complications were recorded for any case.
Demographic and clinical data of the study population are presented in Table 1. There was no statistically significant difference between both the groups with regard to age, sex, weight, ASA PS, duration of surgery, duration of anesthesia and time from premedication to the end of surgery.
Induction was uneventful with only one reported case of laryngospasm and one case of difficult venous access. Those patients were excluded from the study and replaced by two patients fulfilling the inclusion criteria.
It is to be noted that the inhalational anesthetic was switched off at the same timing after skin closure in each group, the best optimal hypotensive anesthesia was equally achieved in both groups.
There were statistically significant differences between patients premedicated with IV midazolam and those of the normal saline group as regards all measured recovery parameters (p < 0.001) (Table 2). Patients in group M scored higher than those in Group S on the OPDS. Yet, this difference did not show statistical significance (p = 0.438) (Table 2).
Among patients premedicated with midazolam, 17 (56.6%) suffered from EA as measured by AFPS compared to 12 (40%) patients from the other group. This difference did not reach statistical significance (p = 0.196) (Fig. 1).
| Characteristics |
Group S (n= 30) |
Group M (n= 30) |
P value |
|---|---|---|---|
| Age (years) | 3.97±0.85 | 3.87±0.94 | 0.667 |
| Sex | – | – | 0.796 |
| Male | 14 (46.7%) | 15 (50%) | |
| Female | 16 (53.3%) | 15 (50%) | |
| Weight (Kg) | 14.07±1.48 | 14.17±1.46 | 0.975 |
| ASA physical status | – | – | 0.542 |
| I | 22 (73.3%) | 24 (80%) | |
| II | 8 (26.7%) | 6 (20%) | |
| Duration of surgery (min) | 106.2±5.59 | 108.98±5.94 | 0.069 |
| Duration of anesthesia (min) | 118.91±4.41 | 121.03±5.39 | 0.1 |
| Time from premedication to end of surgery (min) | 128.91±4.41 | 131.03±5.39 | 0.1 |
| Variables |
Group S (n= 30) |
Group M (n= 30) |
P value |
|---|---|---|---|
| Time to spontaneous eye opening (min) | 10.47±1.17 | 12.53±4.18 | <0.001 |
| Time to react with the nurse or the parents (min) | 20.03±1.33 | 27.93±1.02 | <0.001 |
| Time to attain full modified Aldrete score (min) | 19.77±1.76 | 24.97±2.94 | <0.001 |
| Time to drinking (min) | 42.33±6.69 | 53.8±4.85 | <0.001 |
| Time to hospital discharge (min) | 90.5±11.29 | 99.53±10.52 | <0.001 |
| OPDS | 3 (2-5) | 4 (2-5) | 0.438 |
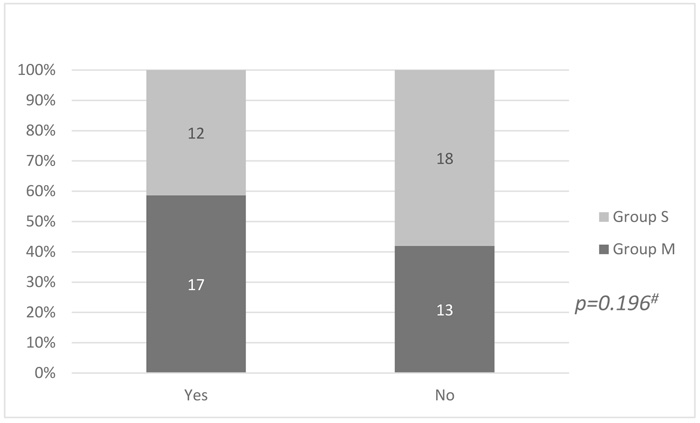 |
Fig. (1). Comparison between the two patients’ groups as regards Emergence Agitation (EA). |
4. DISCUSSION
Oral midazolam premedication is one of the main pharmacological sedations which gained popularity in pediatric anesthesia, especially in ambulatory practice [21]. In our institute, oral midazolam was not available nor was parental attendance allowed. The children received chloral hydrate syrup 30mg/kg half an hour before surgery. However, this was not enough when they were examined in the operating theatre.
The results of the study showed that premedication with IV midazolam in pediatric patients caused a significant delay in both recovery and hospital discharge after moderately lengthy operations under isoflurane anesthesia.
The delay in emergence may have been due to the residual sedative effects of midazolam after a considerable period of giving isoflurane anesthesia; the mean time from premedication to the end of anesthesia was 100 ± 15 minutes in-group M.
Although the maximal sedative effect after IV midazolam occurs after 30 minutes, the serum concentration peaks at 50-60 minutes, and can remain above the suggested therapeutic level until 2 hours after administration [21].
The peak serum midazolam concentration that coincided with the least remaining effect of anesthesia could partly explain the delayed recovery. The concomitant use of isoflurane would have further contributed to this delay, this was not the case in the saline group when the same MAC of isoflurane was achieved and switched off at the same time, so the association of isoflurane with midazolam has been shown to potentiate the effect of one another in inducing unconsciousness.
Similar findings were reported in previous studies in which oral premedication with midazolam delayed both early recovery and hospital discharge when halothane or isoflurane was used for anesthesia maintenance after induction with thiopental or propofol [8, 10, 12, 22].
On the other hand, other studies reported that recovery times were not affected in the same patient group when using inhaled anesthesia with halothane only [6, 8].
Several factors might have contributed to these conflicting results, including the difference in dose, route of administration of midazolam, and the induction technique used. Synergistic interaction with regard to hypnosis has been demonstrated between midazolam and thiopental, propofol, alfentanil and chloral hydrate [23-25]. In the present study, the use of chloral hydrate preoperatively may have also promoted the delay in the group receiving midazolam.
In our study, premedication with IV midazolam has been observed to be associated with higher OPDS score and more patients suffering from EA (56.6%). Yet, these findings did not show statistical significance (p = 0.438 and p = 0.196, respectively). These results could be explained by the fact that no narcotics were provided except during the induction of anesthesia in both groups, and probably its effect declined by the time surgery was completed. The only analgesics administered were paracetamol intraoperative, and diclofenac suppositories at the end. In a meta-analysis conducted by Dahmani and colleagues, in 2010, midazolam was found to be ineffective in the prevention of EA [26]. Similarly, neither IV, oral nor rectal midazolam was found to reduce the incidence of EA in other studies [7, 27, 28]. In a recent study conducted by Kim and colleagues, in 2016, premedication with ketamine was found to be more effective than midazolam in preventing EA during the early emergence period after sevoflurane anesthesia in pediatric patients [29].
On the other hand, another study noted a significantly lower incidence and less severity of sevoflurane-induced EA without delaying discharge from the PACU, in patients pre-medicated with midazolam [30]. In addition, administration of a subhypnotic dose of IV midazolam (0.05 mg/kg), in addition to fentanyl before discontinuation of sevoflurane, was also found to be effective in decreasing EA [31].
There are some limitations in this study that should be noted including the relatively small-sized sample that was restricted to the pediatric age group and the single point assessment of OPDS.
CONCLUSION
Premedication with IV midazolam delayed recovery in pediatric patients undergoing cochlear implants when isoflurane was used as the inhalation anesthetic, while its effect on EA remained uncertain.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The work was approved by the Research Ethics Committee of Ain Shams University Hospital (FMASU R51/ 2018). The study was prospectively registered with the Pan African Clinical Trial Registry (PACTR) with Registration Number PACTR201810739290859.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Pediatric patients were enrolled after informed consent was obtained from their parents or their legal guardians.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
| [1] | Wright KD, Stewart SH, Finley GA, Buffett-Jerrott SE. Prevention and intervention strategies to alleviate preoperative anxiety in children: A critical review. Behav Modif 2007; 31(1): 52-79. |
| [2] | Sheta SA, Alsarheed M. Oral midazolam premedication for children undergoing general anaesthesia for dental care. Int J Pediatr 2009; 2009274380 |
| [3] | Abdallah C, Hannallah R. Premedication of the child undergoing surgery. Middle East J Anaesthesiol 2011; 21(2): 165-74. |
| [4] | Phaltankar J, Shah M. Oral midazolam as a pre-medication in paediatric patients. MVP J Med Sci 2017; 4: 30-54. |
| [5] | Payne K, Mattheyse FJ, Liebenberg D, Dawes T. The pharmacokinetics of midazolam in paediatric patients. Eur J Clin Pharmacol 1989; 37(3): 267-72. |
| [6] | McMillan CO, Spahr-Schopfer IA, Sikich N, Hartley E, Lerman J. Premedication of children with oral midazolam. Can J Anaesth 1992; 39(6): 545-50. |
| [7] | Weldon BC, Watcha MF, White PF, Therapy A. Oral midazolam in children: Effect of time and adjunctive therapy. Anesth Analg 1992; 75(1): 51-5. |
| [8] | McCluskey A, Meakin GH. Oral administration of midazolam as a premedicant for paediatric day-case anaesthesia. Anaesthesia 1994; 49(9): 782-5. |
| [9] | Rosenbaum A, Kain ZN, Larsson P, Lönnqvist PA, Wolf AR. The place of premedication in pediatric practice. Paediatr Anaesth 2009; 19(9): 817-28. |
| [10] | Cray SH, Dixon JL, Heard CM, Selsby DS. Oral midazolam premedication for paediatric day case patients. Paediatr Anaesth 1996; 6(4): 265-70. |
| [11] | Cox RG, Nemish U, Ewen A, Crowe M-J. Evidence-based clinical update: Does premedication with oral midazolam lead to improved behavioural outcomes in children? Can J Anaesth 2006; 53(12): 1213-9. |
| [12] | Viitanen H, Annila P, Viitanen M, Yli-Hankala A. Midazolam premedication delays recovery from propofol-induced sevoflurane anesthesia in children 1-3 yr. Can J Anaesth 1999; 46(8): 766-71. |
| [13] | Chakrabarty A, Tarneja VK, Singh VK, Roy PK, Bhargava AK, Sreevastava DK. Cochlear implant: Anaesthesia challenges. Med J Armed Forces India. Director General, Armed Forces. Med Serv 2004; 60: 351-6. |
| [14] | Pedersen CB, Jochumsen U, Madsen S, Koefoed-Nielsen B, Johansen LV. [Results and experiences with 55 cochlear implantations] Ugeskr Laeger [Internet] 2000; 162: 5346-0. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11036450 |
| [15] | Phoon HYP, Kan AD, Ip Yam PC. A peri-operative audit of the paediatric cochlear implantation programme at the Singapore General Hospital. Proc Singapore Healthc 2013; 22: 131-9. |
| [16] | Aldrete JA, Patel R, Ehrenpreis M, Hannallah R. The post-anesthesia recovery score revisited. J Clin Anesth 1995; 7(1): 89-91. |
| [17] | Norden J, Hannallah R, Getson P, O’Donnell R, Kelliher G, Walker N. Reliability of an objective pain scale in children. J Pain Symptom Manage Elsevier 1991; 6: 196. |
| [18] | Bergendahl HTG, Lönnqvist PA, Eksborg S, et al. Clonidine vs. midazolam as premedication in children undergoing adeno-tonsillectomy: A prospective, randomized, controlled clinical trial. Acta Anaesthesiol Scand 2004; 48(10): 1292-300. |
| [19] | Kim D, Doo AR, Lim H, et al. Effect of ketorolac on the prevention of emergence agitation in children after sevoflurane anesthesia. Korean J Anesthesiol 2013; 64(3): 240-5. |
| [20] | Aono J, Ueda W, Mamiya K, Takimoto E, Manabe M. Greater incidence of delirium during recovery from sevoflurane anesthesia in preschool boys. Anesthesiology 1997; 87(6): 1298-300. |
| [21] | Barkan S, Breitbart R, Brenner-Zada G, et al. A double-blind, randomised, placebo-controlled trial of oral midazolam plus oral ketamine for sedation of children during laceration repair. Emerg Med J 2014; 31(8): 649-53. |
| [22] | Morley-Forster P, McAllister JD, Vandenberghe H, et al. Does thiopentone delay recovery in children premedicated with midazolam? Paediatr Anaesth 1997; 7(4): 279-85. |
| [23] | McClune S, McKay AC, Wright PM, Patterson CC, Clarke RS. Synergistic interaction between midazolam and propofol. Br J Anaesth 1992; 69(3): 240-5. |
| [24] | Short TG, Galletly DC, Plummer JL. Hypnotic and anaesthetic action of thiopentone and midazolam alone and in combination. Br J Anaesth 1991; 66(1): 13-9. |
| [25] | Wollenberg M, Lalwani K. Noct’ Out.A Case Approach to Perioperative Drug-Drug Interactions 2015; 397-400. |
| [26] | Dahmani S, Stany I, Brasher C, et al. Pharmacological prevention of sevoflurane- and desflurane-related emergence agitation in children: A meta-analysis of published studies. Br J Anaesth 2010; 104(2): 216-23. |
| [27] | Breschan C, Platzer M, Jost R, Stettner H, Likar R. Midazolam does not reduce emergence delirium after sevoflurane anesthesia in children. Paediatr Anaesth 2007; 17(4): 347-52. |
| [28] | Cohen IT, Drewsen S, Hannallah RS. Propofol or midazolam do not reduce the incidence of emergence agitation associated with desflurane anaesthesia in children undergoing adenotonsillectomy. Paediatr Anaesth 2002; 12(7): 604-9. |
| [29] | Kim KM, Lee KH, Kim YH, Ko MJ, Jung J-W, Kang E. Comparison of effects of intravenous midazolam and ketamine on emergence agitation in children: Randomized controlled trial. J Int Med Res 2016; 44(2): 258-66. |
| [30] | Ko YP, Huang CJ, Hung YC, et al. Premedication with low-dose oral midazolam reduces the incidence and severity of emergence agitation in pediatric patients following sevoflurane anesthesia. Acta Anaesthesiol Sin 2001; 39(4): 169-77. |
| [31] | Chen J, Li W, Hu X, Wang D. Emergence agitation after cataract surgery in children: A comparison of midazolam, propofol and ketamine. Paediatr Anaesth 2010; 20(9): 873-9. |




