All published articles of this journal are available on ScienceDirect.
Effect of Dexmedetomidine on Postoperative Hemodynamics and Outcome of Free Flaps in Head and Neck Reconstructive Surgeries
Abstract
Background and Aims:
Dexmedetomidine is less frequently used during free flap surgeries for fear of causing vasoconstriction leading to flap failure as it is an α2 agonist. But most patients following major resections with free flap reconstruction develop uncontrolled hypertension postoperatively which could lead to complications resulting in reduced flap perfusion. We aimed to compare the effects of dexmedetomidine on postop haemodynamics, re-exploration rates and final outcome of free flaps in patients who underwent reconstructive surgeries.
Material and Methods:
This prospective, randomized study was conducted in 40 patients aged 20-70 years. Patients in both groups received morphine 0.1mg/kg, 30 minutes before end of surgery. In addition, in Group D dexmedetomidine 1mcg/kg bolus was also given at the same time, followed by 0.5mcg/kg/hr infusion. Post operatively the patients received either dexmedetomidine 0.5mcg/kg/h (Group D) or morphine 2mg/hr (Group M) infusion for 12 hours.
Statistical analysis was done using Chi-Square test and independent sample t test.
Results:
Morphine group had significantly higher heart rate (105.2 ± 7.5 vs 90.0 ± 11.7), systolic blood pressure (167.5 ± 7.3 vs 125.4 ±16.6) and mean arterial pressures (103.5 ± 4.6 vs 87.8 ± 12.2) than dexmedetomidine group. Same trend persisted till 12 hours post operatively. More patients in morphine group required re-exploration of the flap (15 vs 10%) and had flap failure (7.5 vs 2.5%), but these differences were not statistically significant.
Conclusion:
Dexmedetomidine can be safely used in patients with free flap reconstruction as it optimizes postoperative hemodynamics and is not associated with any significant increase in re-exploration or flap failures.
Key Messages:
Dexmedetomidine resulted in optimal postoperative hemodynamics, reduced re-exploration rate and better flap outcome.
INTRODUCTION
Though dexmedetomidine is being commonly used for postoperative sedation, it is less frequently used following free flap surgeries for fear of causing vasoconstriction leading to flap failure as it is an α2 agonist [1]. But the immediate postoperative period in patients who have undergone major head and neck resections with free flap reconstruction is usually stormy with severe hypertension which is difficult to manage. Uncontrolled hypertension could lead to hematoma formation which results in reduced flap perfusion secondary to compression of anastomotic vessels. This is a surgical emergency necessitating immediate re-exploration and any delay could jeopardize the flap survival. So in the present study we assessed the effect of intravenous dexmedetomidine on post operative hemodynamics, possibility of re-exploration and final outcome of free flaps in head and neck surgical patients following major resection and free flap reconstruction.
MATERIAL AND METHODS
No published papers could be located in the existing literature comparing the effect of dexmedetomidine and morphine on the final outcome of free flaps in head and neck surgical patients. So a pilot study was done in 20 patients among whom one half received morphine and the other half received dexmedetomidine in the postop period. The postoperative mean arterial pressure at 30 min after arrival in the postoperative intensive care unit was considered as the primary variable. But because of a wide difference (99.00 ± 5.33 vs 83.30 ± 8.78), even with a confidence interval of 95% and a power of 99%, the calculated sample size was only 8 for each group. However, we recruited 40 patients of both sexes, aged 20-70 years, of American Society of Anesthesiologists physical status I-III undergoing major head and neck resection surgeries with free flap reconstruction. The study was conducted from January 2014 to December 2014 after obtaining ethics committee approval and patients’ consent.
Patients with uncontrolled hypertension, congestive heart failure, renal or hepatic impairment, hyperthyroidism, history of hypersensitivity to the test drugs and those on oral morphine or antipsychotics were excluded.
Patients were randomly allocated to one of the two groups using computer generated sequence of random numbers to group M and group D. All patients received a standardized anesthesia management and were induced with glycopyrrolate 0.2mg, midazolam 2mg, morphine 0.2mg/kg, and thiopentone 5mg/kg body weight intravenously (IV). After securing the airway with an endotracheal tube following suxamethonium 2mg/kg, pancuronium 0.1mg/kg was given intravenously. Intraoperative monitoring included electrocardiogram, pulse-oximeter, invasive arterial blood pressure, end tidal carbondioxide and temperature monitoring. Anesthesia was maintained with oxygen-nitrous oxide (1:2) with isoflurane (1-1.5%) and intermittent muscle relaxants. Top up doses of morphine 0.1mg/kg was given every 4th hourly during the surgery. Intraoperative fluids comprised of crystalloids, colloids and packed red cells and an urine output of 0.5ml/kg body weight was ensured.
Half an hour before the end of surgery a bolus of 0.1mg/kg of morphine was given to both groups of patients. For patients allocated in Group D, a bolus of dexmedetomidine 1mcg/kg over 10 minutes was given half an hour before the proposed end of surgery, followed by 0.5mcg/kg/hr infusion. At end of surgery, following tracheostomy, all anaesthetic gases were discontinued and patients were shifted to post operative intensive care unit (ICU) for overnight ventilation. Tracheostomy and overnight ventilation were done for all patients as the surgeries were done on oral cavity or face and any need for reintubation either for reexploration or for management of compromised airway would be extremely difficult. Post operatively Group D received dexmedetomidine 0.5mcg/kg/hr and patients in Group M received morphine 2mg/hr as intravenous infusion for 12 hours in the ICU.
Heart rate (HR) and blood pressure (BP) were recorded at end of surgery, on arrival in the ICU, then every half hourly up to 4 hours, then every hour till 8 hours followed by every 2 hourly readings till 12 hours. If patients were found to have tachycardia (HR > 100/min) or elevated blood pressure (systolic BP > 140mmHg), midazolam 1-2mg bolus was given IV initially. If neither of the variables decreased, metoprolol 1mg every 15min was given, up to maximum of 5mg IV. If HR and BP are still found to be elevated, nitroglycerine infusion was started and titrated to maintain an SBP of 110-130mmHg.
Though the hemodynamic study period lasted only for 12 hours, patients were monitored for re-exploration of the flap in the first postoperative day and the viability of the free flap at discharge from hospital was also documented.
IBM Statistical Package for Social Sciences (SPSS) version 20.0 was used for data analysis. Chi-Square test was used to find the association of re-exploration and flap outcome. Independent sample t test was used to compare demographic and hemodynamic variables among the groups. Significance was assumed for p values <0.05.
RESULTS
The demographic variables were comparable between the groups. At the end of the surgery, statistical analysis revealed that morphine group had significantly higher heart rate (105.2 ± 7.5 vs 90.0 ± 11.7), systolic BP (167.5 ± 7.3 vs 125.4 ± 16.6) and mean arterial pressures (103.5±4.6 vs 87.8±12.2) than dexmedetomidine group (p <0.001). The same trend persisted throughout the post operative period up to 12 hours, with morphine group showing significantly higher hemodynamic parameters (Table 1, Figs. 1 & 2).
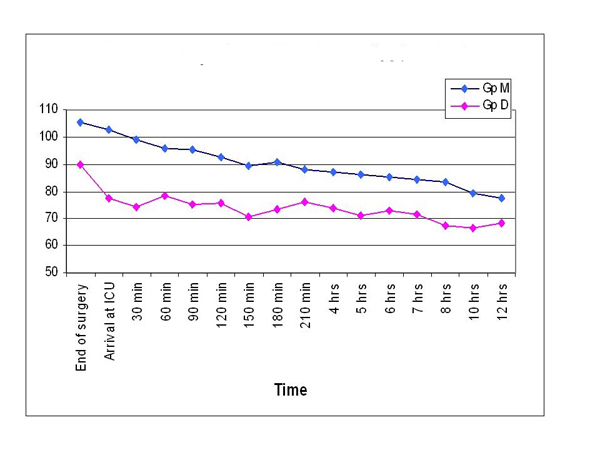
Comparison of postoperative heart rates.
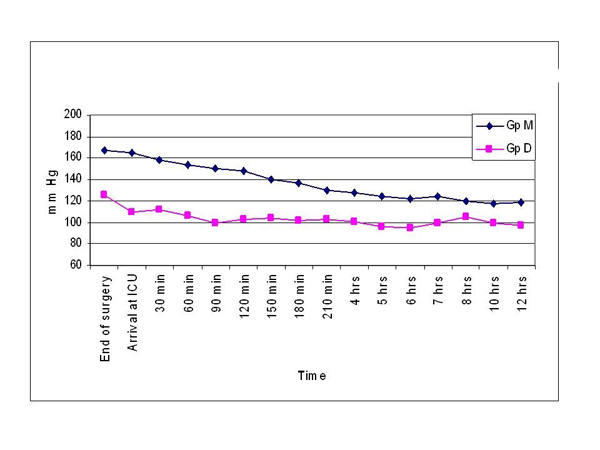
Comparison of systolic BP.
Comparison of postoperative mean arterial pressures.
| Time | Groups | p value | |||
|---|---|---|---|---|---|
| Morphine (n 20) | Dexmedetomidine (n 20) | ||||
| Mean | SD | Mean | SD | ||
| End of surgery | 103.5 | 4.6 | 87.8 | 12.2 | <0.001* |
| Arrival at ICU | 102.2 | 3.3 | 84.7 | 7.1 | <0.001* |
| 30 min | 97.6 | 4.0 | 82.3 | 6.8 | <0.001* |
| 60 min | 94.3 | 3.8 | 80.1 | 7.2 | <0.001* |
| 90 min | 92.2 | 3.4 | 75.3 | 7.2 | <0.001* |
| 120 min | 92.1 | 3.1 | 74.8 | 9.0 | <0.001* |
| 150 min | 88.5 | 4.2 | 74.7 | 8.2 | <0.001* |
| 180 min | 84.1 | 4.7 | 75.3 | 8.5 | <0.001* |
| 210 min | 84.3 | 4.2 | 71.3 | 6.8 | <0.001* |
| 4 hours | 80.5 | 5.8 | 72.8 | 5.4 | <0.001* |
| 5 hours | 78.1 | 5.0 | 69.6 | 7.4 | <0.001* |
| 6 hours | 76.5 | 4.4 | 73.1 | 7.6 | 0.017* |
| 7 hours | 73.4 | 4.3 | 68.4 | 6.0 | <0.001* |
| 8 hours | 70.6 | 4.3 | 67.4 | 6.5 | 0.010* |
| 10 hours | 72.8 | 4.0 | 67.7 | 5.8 | <0.001* |
| 12 hours | 70.4 | 4.4 | 69.3 | 5.1 | 0.0327* |
More number of patients in the morphine group required re-exploration of the flap (15 vs 10%), but the difference was not statistically significant (p value 0.737, Table 2). Similarly, though the flap failure rate was more in morphine group compared to dexmedetomidine group (7.5 vs 2.5%), there was no statistically significant difference between the groups (p value 0.615, Table 3).
Comparison of re-exploration.
| Groups | No | Yes | p value |
|---|---|---|---|
| n (%) | n (%) | ||
| Morphine | 34 (85.0) | 6 (15.0) | 0.737 |
| Dexmedetomidine | 36 (90.0) | 4 (10.0) |
Comparison of flap outcome.
| Groups | Successful | Failure | p value |
|---|---|---|---|
| n (%) | n (%) | ||
| Morphine | 37 (92.5) | 3 (7.5) | 0.615 |
| Dexmedetomidine | 39 (97.5) | 1 (2.5) |
DISCUSSION
In view of better functional outcomes and improved aesthetics, microvascular free flaps are preferred for most major head and neck reconstructive surgeries [2]. Reconstructive free flap surgery involves the transfer of free tissue, comprising of skin, muscle, bone, bowel or a combination of these, to a large surgical defect not amenable to primary closure. As the circulation to a free flap is through micro vascular anastamoses, maintaining adequate perfusion is essential for a successful outcome.
The success of free flap reconstruction greatly depends on good surgical technique as well as on vigilant postoperative care and monitoring [3]. The common vascular causes of free flap failure is venous (35%), arterial (28%), hematoma formation (26%) and recipient vessel problems (11%) [19]. Though it is essential to avoid hypotension postoperatively following free flap surgeries, uncontrolled hypertension can also be equally dangerous. Uncontrolled hypertension in the immediate postoperative period could lead to increased bleeding from the surgical site, even if there was adequate hemostasis intraoperatively. The effect of the resultant hematoma could have a devastating effect on the perfusion of the free flap. Extrinsic compression by hematoma can progress rapidly to arterial thrombosis and ultimately to flap failure [3].
In the intraoperative period, following perfusion of the free flap, controlled hypertension along with haemodilution is preferred so as to improve the perfusion of the flap across the microvascular anastomoses. The probable reason for the severe hypertensive response commonly observed in the immediate postoperative period could be secondary to the intraoperative volume expansion. At the end of surgery, with discontinuation of the inhalational agents, the vascular tone is regained and hypertension could result even in the presence of adequate analgesia.
Very often the postoperative hypertension following free flap reconstruction ultimately necessitates use of vasodilators like nitroglycerin. But this could result in sudden and significant fall in blood pressure with a reduction in the flap perfusion. The problem with many other commonly used antihypertensive drugs is that the duration of action is prolonged. So if we can avoid this sudden surge in BP in the immediate postop period, to some extent it may be possible to reduce re-explorations for hematoma evacuation. This is important because, though early free flap pedicle failure does not necessarily equate to complete flap loss, [4] use of a second free flap later for the defect correction becomes technically more demanding. Morphine is the time tested drug which is commonly used for postoperative analgesia and sedation. But blood pressure as measured by frequency of variation was less stable in patients who received morphine in the immediate postoperative period of head and neck reconstructive surgery [5]. An opioid agonist with primary actions of analgesia and sedation, intravenous morphine has vasodilator effect and acts particularly on the venous system. It has little or no effect on mean arterial blood pressure but improves systemic blood flow by reducing vascular resistance.
A major drawback of morphine is that the time of onset and peak action are delayed even when given intravenously. The main factor responsible for its latency is low lipid solubility and slow penetration of blood brain barrier, making it less suitable in the management of sudden hypertension of the immediate post operative period. Due to delayed action overdosing can occur and once hypotension sets in, it is usually prolonged.
Dexmedetomidine, a short acting α2 agonist with sedative, anxiolytic, analgesic, neuroprotective and anaesthetic sparing properties, [6] is increasingly being used as an adjunct to both regional [7] and general anaesthesia [8] and also as a postoperative sedative [9]. It has been safely used for postoperative sedation following neurosurgical [10] and cardiovascular procedures [11]. The administration of dexmedetomidine before the completion of major surgical procedures was found to be associated with better hemodynamic stability in the postoperative period [12].
Despite its wide spread perioperative use, there is a certain reservation for its use following free flap surgeries. Being an α2 agonist it is expected to induce vasoconstriction in denervated flaps [1] with a subsequent hypoperfusion of the free flap. Following a restricted arterial flow, the glucose concentration decreases in the flap muscle with an elevation of lactate in all flap components. The lactate-to-pyruvate ratio remains normal during arterial obstruction but with venous obstruction it increases [13]. But it has been demonstrated that even if dexmedetomidine is used for deep sedation, it does not affect the perfusion or metabolism in denervated musculocutaneous flaps as measured by lactate-pyruvate and lactate-glucose ratios monitored by microdialysis and by tissue-oxygen partial pressure [14].
Moreover the direct effect of dexmedetomidine on the peripheral vascular smooth muscle doesn’t usually last >10 min and this effect can be attenuated by a slow infusion over 10 min or more [15, 16]. In addition, the vasodilatory actions of morphine, [17] if used simultaneously, may obtund the vasoconstrictor effects of dexmedetomidine. Safe use of the drug following coronary artery bypass grafts [18, 11] also suggests that dexmedetomidine when used as a postoperative sedative does not result in clinically significant vasoconstriction of the denervated vasculature. Based on these evidences the perioperative use of dexmedetomidine in free flap reconstructive surgeries is justified.
In the present study it was seen that postoperative hemodynamics were well controlled in the dexmedetomidine group with reduced re-exploration rates, which got reflected in the flap outcome also. The heart rate and blood pressure being significantly different from the end of surgery itself could be because the dexmedetomidine group received the drug 10 minutes before end of surgery and the drug effect had already set in.
Though we observed reduced rates of re-exploration and better flap outcome in the dexmedetomidine group, the statistical analysis showed it to be insignificant. This could be because our study was probably underpowered for assessment of the final outcome of free flaps and a higher sample size might prove the difference to be significant.
CONCLUSION
Dexmedetomidine can be safely used in head and neck surgical patients with free flap reconstruction as it optimizes postoperative hemodynamics and is not associated with any significant increase in re-exploration or flap failure. Though not statistically significant, the administration of dexmedetomidine in our study resulted in reduced re-exploration rates and better flap outcome.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


