All published articles of this journal are available on ScienceDirect.
Should Low Central Venous Pressure Be Maintained during Liver Transplantation?
Abstract
Low central venous pressure, which indirectly reflects free hepatic venous pressure, is maintained during hepatic resection surgery to reduce intraoperative blood loss by facilitating hepatic venous outflow. However, whether the low central venous pressure protocol established for non-transplant hepatobiliary surgery should be generalized to liver transplantation is controversial because patients with cirrhosis have decreased portal and hepatic venous blood flow and vulnerability to renal failure. However, consistent with observations from hepatic resection surgeries, lowering central venous pressure during the preanhepatic phase significantly reduces blood loss and transfusion volume. Conversely, inherent study limitations and different study designs have yielded different results in terms of renal dysfunction. Although hepatic venous outflow promoted by lowering blood volume seems to facilitate a liver graft to accommodate portal blood flow increased by portal hypertension-induced splanchnic vasodilatation, the association between low central venous pressure and reduced incidence of portal hyperperfusion injury has not been demonstrated. Stroke volume variation predicts fluid responsiveness better than central venous pressure, but it has not been associated with a greater clinical benefit than central venous pressure to date. Therefore, the safety of maintaining low central venous pressure during liver transplantation has not been verified, and further randomized controlled studies are warranted to establish a fluid management protocol for each phase of liver transplantation to reduce intraoperative blood loss and transfusion rate, thereby maintaining liver graft viability. In conclusion, low central venous pressure reduces intraoperative blood loss but does not guarantee renoprotection or graft protection.
INTRODUCTION
Maintenance of low central venous pressure has been advocated for hepatic resection surgery because it facilitates hepatic venous outflow, which decreases the resistance of blood flow from the hepatic venous system into the inferior vena cava and prevents hepatic congestion, thereby decreasing bleeding from the sinusoids or hepatic veins during surgery [1]. Based on this mechanism, several methods for maintaining low central venous pressure during liver transplantation have been reported, with non-uniform results [2-5]. Hence, whether to generalize the protocol used for non-transplant hepatobiliary surgery to liver transplantation has been addressed based on different physiological factors with respect to the liver [6]. Recently, an optimal range of central venous pressure during the neohepatic phase was suggested to reduce portal hyperperfusion following liver graft reperfusion; notably, this range would not be considered low compared to the central venous pressure levels that have been maintained in previous studies. Such portal hyperperfusion occurs due to the persistent hyperdynamic splanchnic circulation that is present in patients with cirrhosis who undergo liver transplantation and is complicated by portal hypertension [7]. In addition, accumulating evidence has shown the superiority of stroke volume variation compared to central venous pressure in terms of assessing fluid responsiveness in patients undergoing liver transplantation [8-11]. In this regard, the current review discusses the safety of maintaining low central venous pressure during liver transplantation based on hepatic physiology, liver cirrhosis pathophysiology, and results from previous studies.
CENTRAL VENOUS PRESSURE, A FEASIBLE SURROGATE FOR FREE HEPATIC VENOUS PRESSURE
After the induction of anesthesia for liver transplantation, the subclavian or internal jugular veins are frequently catheterized and sometimes the femoral vein is also catheterized. A central venous catheter tip is generally inserted via the subclavian or internal jugular vein at the junction of the right atrium and superior vena cava or in the lower third of the superior vena cava [12]. The pressure measured from the tip of this catheter is considered the central venous pressure and is a key physiologic estimate of preload, which can help determine intravascular fluid status. Central venous pressure also reflects free hepatic venous pressure because the hepatic veins lie within 0.5 to 3.0 cm of the ostium of the right atrium [13]. In addition, no significant anatomical structure prevents the patency from the hepatic veins to the lower portion of the superior vena cava. Although a Eustachian valve originating from the inferior vena cava orifice can exist, it does not influence the venous flow from the inferior vena cava to the right atrium, except for in rare extreme cases [14]. Unless there is a positional change, such as head-down or head-up tilt, the consistency between central venous pressure and free hepatic venous pressure is not influenced by positive end-expiratory pressure [15]. Moreover, central venous pressure has a linear relationship with portal venous pressure [16, 17].
However, when the tip of a catheter is placed through the femoral vein, it usually does not reach the inferior vena cava. As such, there is a longer distance between the catheter tip and the hepatic veins than when a catheter tip is introduced from the subclavian or internal jugular vein. In addition, because there is a greater dynamic pressure gradient between the hepatic veins and femoral veins than that between the hepatic veins and superior vena cava [18], inaccuracy can arise when using a catheter inserted from the femoral vein for the indirect measurement of free hepatic venous pressure. Furthermore, the pressure measured from the catheter tip is affected by manipulation of the liver and total or partial clamping of the infrahepatic inferior vena cava, which can impede venous return from the infrahepatic inferior vena cava or hepatic veins to the right atrium, resulting in increased pressure (Fig. 1). Therefore, measurements of central venous pressure from the superior vena cava or the junction of the right atrium and superior vena cava indirectly reflect free hepatic venous pressure and can help clinicians assess resistance to hepatic venous outflow.
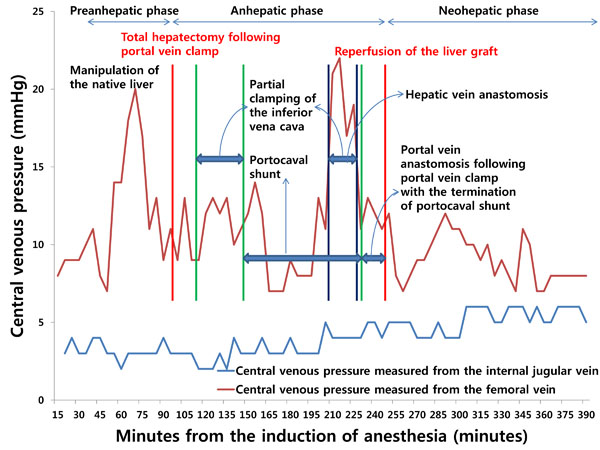
INFLUENCES OF DIFFERENT HEPATIC HEMODYNAMICS AND SURGICAL PROCEDURES ON LOW CENTRAL VENOUS PRESSURE IN PATIENTS UNDERGOING HEPATIC RESECTION VERSUS LIVER TRANSPLANTATION
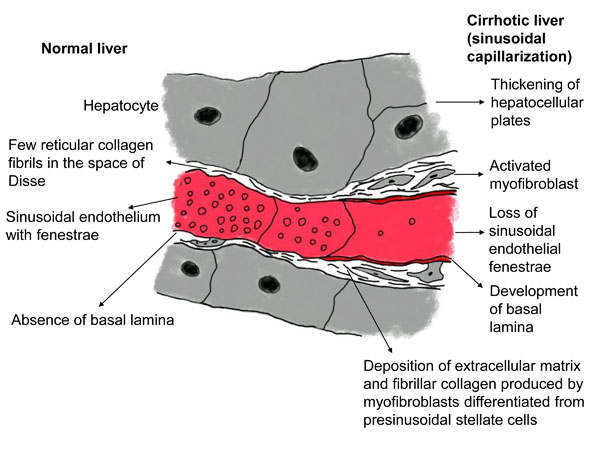
The liver is perfused by the hepatic artery and portal vein and is drained by the hepatic veins. The pressure gradient between the inflow and outflow of the liver (i.e., the hepatic venous pressure gradient) is 1 to 5 mmHg [19]. In a cirrhotic liver, an increase in portal tract extracellular matrix with sclerosis around the portal veins increases presinusoidal vascular resistance. The development of abnormal hepatic artery-to-portal vein shunts results in an increase in portal venous pressure (Fig. 2). In the sinusoids, the endothelial fenestrae are lost with the development of the basal lamina and the expansion of fibrosis in the space of Disse, leading to an increase in sinusoidal resistance (Fig. 3). Increased post-sinusoidal resistance due to sclerosis around the terminal hepatic veins impedes the outflow of sinusoidal blood (Fig. 2). The net results of the impaired hepatic blood flow through the liver parenchyma are portal hypertension, characterized by an increased hepatic venous pressure gradient of more than 5 mmHg [20]; subsequent development of esophageal varices; and rupture of these varices [21]. In accordance with the above pathophysiology, hepatic venous outflow is also reduced [22, 23].

Hence, the additive or synergistic effects of the reduced hepatic venous outflow and lowering central venous pressure which promotes uninterrupted hepatic venous outflow would be expected during liver transplantation for the treatment of liver cirrhosis. During a hepatectomy, surgeons often resect a physiologically functioning liver with intact hepatic venous outflow. In this context, lowering central venous pressure has a beneficial effect on intraoperative blood loss due to the facilitation of hepatic venous outflow and the consequent prevention of hepatic congestion [24-28]. However, one concern regarding the maintenance of low central venous pressure in patients with cirrhosis who are undergoing liver transplantation is associated with the proneness of such patients to renal failure due to hypovolemia, which can be induced by a decrease in central venous pressure. The potential for this relationship exists due to such patients’ underlying medical conditions, which can include circulatory dysfunction with arterial underfilling, increased endogenous vasoconstrictor activity that affects intra-renal circulation, and increased systemic inflammatory responses [29].
CLINICAL IMPLICATIONS OF MAINTAINING LOW CENTRAL VENOUS PRESSURE DURING LIVER TRANSPLANTATION
Notwithstanding the risk for renal failure, a low central venous pressure protocol has been used in several studies of liver transplantation (Table 1). PubMed, EMBASE, and Cochrane library were searched for the studies relevant to this review published in English from 2000 to August 2016, using the Medical Subject Headings which were central venous pressure and liver transplantation. Studies that investigated the clinical outcomes of central venous pressure maintained low during liver transplantation in adult patients were included. Since there were only a few prospective studies, retrospective studies were also included in this review. Then, data were abstracted on study design, sample size per group, target range and strategies for low central venous pressure, clinical outcomes, and study limitations.
The first published retrospective study showed that patients undergoing liver transplant whose central venous pressure was maintained below 5 mmHg during the preanhepatic (before total hepatectomy of the native liver) and neohepatic (after reperfusion of the liver graft) phases and as low as possible during the anhepatic phase (when vascular anastomoses of the liver graft are performed) by minimal administration of fluid and use of alpha-agonists (phenylephrine or norepinephrine), venodilators (nitroglycerin), morphine, and furosemide, were transfused less than patients whose central venous pressure was maintained at normal levels. However, the former group of patients required more postoperative dialysis, showed higher levels of peak creatinine and had a higher 30-day mortality rate [5]. Notably, in the referenced study, two different protocols (low central venous pressure versus normal central venous pressure) were employed in two different medical centers, and the preoperative medical status of the normal central venous pressure group was poorer than that of the low central venous pressure group. Hence, a clear conclusion could not be drawn. A few years later, contrasting results were produced in another study, in which a 40% decrease in baseline central venous pressure caused by restriction of fluid infusion and/or phlebotomy without volume replacement during the preanhepatic phase reduced transfusion quantity without causing a significant difference in serum creatinine value on the 5th postoperative day or in 1-year mortality [4]. Later, more subjects were recruited for the low central venous pressure group, and the results became more favorable. Specifically, the low central venous pressure group had significantly lower serum creatinine levels 1 year after liver transplantation compared to the control group [3]. A limitation of the referenced study is that the control group data were obtained retrospectively, whereas the low central venous pressure group data were collected prospectively. In addition, the average duration of surgery was approximately 255 minutes because the surgeons were considerably proficient, indicating that a low central venous pressure protocol should be used with caution in transplant centers that have not reached a learning curve plateau. In a recent prospective randomized controlled study [2], central venous pressure was reduced to below 5 mmHg or by 40% of the baseline value during the preanhepatic phase by minimizing infusion volume, adjusting posture with a head-up tilt, and administering somatostatin and nitroglycerin. This low central venous pressure resulted in significant decreases in blood loss and transfusion quantity, as well as lower lactate levels at the end of surgery and better preservation of hepatic function after liver graft reperfusion. Furthermore, no significant differences in renal function or incidence of postoperative complications were observed between the treatment groups. In another prospective randomized controlled parallel study [30], maintenance of central venous pressure below 5 mmHg or lower than the baseline by about 40% by the Trendelenberg position, limiting infusion volume, and administration of nitroglycerine and furosemide also reduced the amount of intraoperative blood loss and transfusion. Interestingly, the low central venous pressure decreased the incidence of postoperative pulmonary complications (i.e., pleural effusion, pulmonary infection, and pulmonary edema) as well as mechanical ventilation time. In addition, the total urine volume was comparable between the 2 groups (low central venous pressure and control groups).
| References | Study Design | Number of Patients per Group | Target Value of CVP per Phase* | Strategies to Maintain Low CVP | Favorable Clinical Outcomes | Unfavorable Clinical Outcomes | Study Limitations |
|---|---|---|---|---|---|---|---|
| Schroeder et al. (2004) [5] | Retrospective analysis |
Low CVP group: 73 Normal CVP group: 78 |
Preanhepatic and neohepatic phases: < 5 mmHg Anhepatic phase: as low as possible |
Alpha-agonists (phenylephrine or norepinephrine) Venodilator (nitroglycerin) Opioid (Morphine) Diuretic (Furosemide) |
Reduced transfusion rate | Higher peak creatinine level More frequent need for postoperative dialysis Higher 30-day mortality Lower mean arterial pressure during the anhepatic phase |
Patient allocation to groups according to transplant center (low CVP group from transplant center 1; normal CVP group from transplant center 2) Higher proportion of critically ill patients in the normal CVP group Insignificant difference in CVP between the groups during the preanhepatic phase; Significantly low CVP during the anhepatic phase in the normal CVP group compared to the low CVP group (assumed to be an error in data input); Average CVP values (10 to 12 mmHg) close to upper normal limits during the preanhepatic and neohepatic phases |
| Massicotte et al. (2006) [4] | Prospective study combined with retrospective analysis | Prospective low CVP group: 98 Historical control: 206 |
Preanhepatic phase: approximately 60% of the baseline | Restriction of volume infusion Phlebotomy without volume replacement |
Reduced transfusion rate, blood loss, and 72-hour postoperative creatinine level | - | Use of historical controls Short average duration of surgery (approximately 260 minutes) complicating universal application of the protocol to transplantation centers not reaching a learning curve plateau |
| Massicotte et al. (2008) [3]† | Prospective study combined with retrospective analysis | Prospective low CVP group: 300 Historical control: 206 |
Preanhepatic phase: approximately 67% of the baseline | Restriction of volume infusion Phlebotomy without volume replacement |
Reduced transfusion rate, blood loss, and 72-hour and 1-year postoperative creatinine level Higher final hemoglobin level |
- | Use of historical controls Short average duration of surgery (approximately 260 minutes) complicating universal application of the protocol to transplantation centers not reaching a learning curve plateau |
| Feng et al. (2010) [2] | Prospective, randomized-controlled, parallel-group study | Low CVP group: 43 Control group: 43 |
Preanhepatic phase: < 5 mmHg or 60% of the baseline | Restriction of volume infusion Reverse Trendelenberg position Somatostatin Nitroglycerin |
Reduced transfusion rate Lower levels of AST, ALT, TB, and lactate during the neohepatic phase |
Lower mean arterial pressure 2 hours after the surgery | - |
| Cywinski et al. (2010) [32] | Retrospective analysis | Low CVP group: 56 High CVP group: 88 |
Neohepatic phase: < 10 mmHg | Discretion of the attending anesthesiologists | - | Slow rates of decrease in ALT and bilirubin levels between postoperative days 1 and 5 | Significantly low BMI, short case duration, high preoperative platelet count, and high frequency of epinephrine use in the low CVP group |
| Wang et al. (2013) [30] | Prospective, randomized-controlled, parallel-group study | Low CVP group: 33 Control group: 32 |
Approximately 60% of the baseline (the phase in which low CVP was maintained was not specified) | Restriction of volume infusion Trendelenberg position Nitroglycerin Furosemide |
Reduced transfusion rate and blood loss Low incidence of pulmonary complications Early weaning from mechanical ventilation |
- | - |
When a liver graft is reperfused, impaired portal venous blood flow is restored. After reperfusion, a liver graft should accommodate portal venous blood flow, which was already increased (portal hyperperfusion) due to persistent hyperdynamic splanchnic circulation caused by portal hypertension-induced splanchnic vasodilatation and subsequent development of extensive collaterals [31]. Theoretically, if hepatic venous outflow is impaired for any reason, including increased intravascular volume (represented by a high central venous pressure level), then portal hyperperfusion injury would develop and deteriorate. However, a recent retrospective study showed no significant difference in overall patient survival, graft survival or length of intensive care unit and hospital stay between two groups whose central venous pressures were maintained either below 10 mmHg or above 10 mmHg during the neohepatic phase [32]. Concordant with these results, the maintenance of a central venous pressure between 5 to 10.5 mmHg, which is not as low as the central venous pressure that has been maintained in previous studies, after liver graft reperfusion was found to prevent portal hyperperfusion in the early postoperative period [7].
In summary, the use of a low central venous pressure protocol during the preanhepatic phase reduces blood loss and improves transfusion rate, but the effects of this approach on renal complications remain controversial. Despite the potential for portal hyperperfusion injury due to impaired hepatic venous outflow arising from high blood volume, which is represented by high central venous pressure, the maintenance of low central venous pressure during the neohepatic phase does not seem to enhance graft protection.
PERIOPERATIVE RENAL PROTECTIVE STRATEGIES
Because various intraoperative events, such as hemodynamic instability, volume loss, and hemorrhage, contribute to the occurrence of postoperative acute kidney injury [33, 34], diagnosis of acute kidney injury and renal protection are considerably challenging during liver transplantation. Furthermore, there is a lack of substantial data on perioperative renal protective strategies [35]. Compared to the renal dose of dopamine (2 – 3 µg/kg/min), fenoldopam 0.1 µg/kg/min administered for 2 or 4 days from anesthesia induction reduced the postoperative increase rates of serum creatinine and blood urea nitrogen and frequency of furosemide use [36] and maintained creatinine clearance rate unchanged [37]. On the contrary, N-acetylcysteine failed to reduce the incidence of postoperative acute kidney injury [38]. Although equivalent renoprotective effects were shown between hydroxyethyl starch and human albumin in a prospective randomized study [39], a recent retrospective study demonstrated the association of the use of hydroxyethyl starch with the development of acute kidney injury [40]. In addition, osmotic-nephrosis-like lesions of the distal and proximal tubules were observed up to 10 years after the use of hydroxyethyl starch during liver transplantation [41]. Moreover, a black box warning for hydroxyethyl starch use in critically ill patients including liver transplantation recipients was issued by the United States Food and Drug Administration. Chloride-liberal fluids, such as 0.9% saline and 5% albumin, were found to be more associated with an increased risk for postoperative acute kidney injury, than with chloride-restrictive fluids, such as 0.45% saline and Ringer’s lactate [42].
The incidence of acute kidney injury was higher in patients undergoing conventional technique (total occlusion of the inferior vena cava with or without venovenous bypass) than in those undergoing piggyback technique (62% in conventional technique group without venovenous bypass versus 18% in piggyback technique group [P = 0.001] [43]; 21% in conventional technique group using venovenous bypass versus 0% in piggyback technique group [P < 0.05] [44]). However, no significant difference in the incidence of acute kidney injury was observed between conventional technique groups with and without venovenous bypass [45, 46]. However, piggyback technique using temporary portocaval shunt produced beneficial effects on renal function (maintenance of urine output during the anhepatic phase and stable postoperative serum creatinine levels) compared to piggyback technique in the absence of temporary portocaval shunt [47].
In summary, the use of hydroethyl starch and chloride-liberal fluids should be avoided whereas fenoldopam, venovenous bypass during total occlusion of the inferior vena cava, and temporary portocaval shunt are renoprotective in liver transplantation.
DYNAMIC PRELOAD INDICES AND TRANSESOPHAGEAL ECHOCARDIOGRAPHY FOR LIVER TRANSPLANTATION
Although central venous pressure gives an estimate of hepatic venous pressure and indirectly reflects portal venous pressure, recommendations have been made to abandon the static preload index due to its unreliability in assessing intravascular volume status [48, 49]. The static preload indices, which also include pulmonary artery occlusion pressure, failed to predict ventricular filling volume, cardiac performance, or hemodynamic response to volume challenge in normal subjects or septic patients [50, 51]. In particular, the incidence of severe ventricular arrhythmia is high during the insertion and removal of a pulmonary catheter in liver transplant recipients [52]. Hence, dynamic preload indices, such as systolic pressure variation, pulse pressure variation, and stroke volume variation, which represent the fluctuation of systolic pressure, pulse pressure, and stroke volume, respectively, according to a periodic change in intrathoracic pressure during the respiratory cycle under mechanical ventilation, were developed. These indices can be used to represent intravascular volume status via a Frank-Starling curve.
Particularly, stroke volume variation became popular following the introduction of the FloTrac/Vigileo system (Edwards Lifesciences, Irvine, CA, United States). Despite that patients with cirrhosis show altered circulation patterns (increased cardiac output and decreased peripheral vascular resistance) [53, 54], which may affect the accuracy of estimating vascular compliance and resistance and result in an incorrect calculation of stroke volume when using the FloTrac/Vigileo system, stroke volume variation is still a better predictor of fluid responsiveness compared to central venous pressure and pulmonary artery occlusion pressure [9, 55] and its performance is comparable to pulse pressure variability. In addition, it has a better correlation with right ventricular end-diastolic volume index than central venous pressure or pulmonary artery occlusion pressure [10]. Moreover, stroke volume variation is not influenced by positive end-expiratory pressure [8] and the type of artery catheterized for its measurement [56]. In contrast, pulse pressure variability was found to be unreliable predictor of fluid responsiveness during orthotopic liver transplantation [57]. However, the results of the study are not reliable because the study has some inherent limitations. The amount of fluid (350 ml) used for fluid challenge was not enough to increase the stroke volume index by 10% (the criterion for fluid responsiveness) and the study protocol was completed in only 12 (80%) and 7 (46%) patients out of 15 patients during the anhepatic and neohepatic phases, respectively, resulting in less statistical power. To the best of my knowledge, there is no available study which investigated the utility of systolic pressure variability in liver transplantation.
Despite its better performance for fluid management, the use of stroke volume variation did not decrease the incidence of acute kidney injury or of 30-day and 1-year mortality compared to the use of central venous pressure [11]. In addition, unlike with central venous pressure, assessing intravascular volume status by measuring stroke volume variation was not associated with a postoperative decrease in portal hyperperfusion [7]. Furthermore, the role of stroke volume variation in reducing intraoperative blood loss has not been evaluated.
Compared to the year 2002 when only 21% of high-volume centers used transesophageal echocardiography during liver transplantation [58], the percentage of the use of transesophageal echocardiography has increased to 86% in 2008 [59]. It provides visual information about the structural nature, dynamic function, volume status, regional wall motion, and contractility of the heart, pericardial effusion, and embolization of major vessels [60]. In a variety of clinical situations, left ventricular end-diastolic area index, which represents left ventricular filling, was shown to be correlated with stroke volume index (preload) during volume therapy in the absence of the changes in the left ventricular compliance and contractility [61-63]. However, left ventricular end-diastolic area index was not correlated with stroke volume index in patients undergoing liver transplantation [64]. Although its echocardiographic view and planimetry tracing is simple, there are low repeatability and high interindividual variation between operators [63]. In addition, the posterior retraction of the stomach for liver transplantation prevents obtaining an optimal transgastric view [60]. Therefore, the usefulness of left ventricular end-diastolic area index obtained with transesophageal echocardiography for the prediction of fluid responsiveness should be further investigated.
CONCLUSION
Maintaining low central venous pressure, which reflects various hepatic hemodynamic parameters, is useful for reducing intraoperative blood loss during the preanhepatic phase of liver transplantation and during hepatic resection. This relationship holds true despite the differences in hepatic physiology and operative techniques between the two surgeries. However, the renal complications that can potentially result from hypovolemia associated with low central venous pressure must be considered. In addition, low central venous pressure does not guarantee liver graft protection during the neohepatic phase. Manipulation of stroke volume variation produces superior results compared to manipulation of central venous pressure in terms of fluid responsiveness, but it has not been demonstrated to improve clinical outcomes, such as mortality, or to prevent portal hyperperfusion. At present, there is no established protocol for maintaining an appropriate intravascular volume during liver transplantation due to the inherent limitations of previous studies (e.g., small sample size that decreases the statistical power of the studies), which prevents universal extrapolation of the protocol used in the studies. Furthermore, central venous pressure is still recommended to be abandoned [49]. Therefore, further prospective randomized controlled studies assessing each phase of liver transplantation are warranted to identify the optimal central venous pressure or stroke volume variation levels to minimize intraoperative blood loss, improve transfusion rate, and ameliorate postoperative complications, with an overall aim of maintaining liver graft viability. In conclusion, maintaining a low central venous pressure has beneficial effects in liver transplantation while renal protection should always be accompanied.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


