REVIEW ARTICLE
Ventilation via Narrow-Bore Catheters: Clinical and Technical Perspectives on the Ventrain Ventilation System
D. John Doyle1, 2, *
Article Information
Identifiers and Pagination:
Year: 2018Volume: 12
First Page: 49
Last Page: 60
Publisher ID: TOATJ-12-49
DOI: 10.2174/2589645801812010049
Article History:
Received Date: 6/7/2018Revision Received Date: 3/9/2018
Acceptance Date: 4/9/2018
Electronic publication date: 27/09/2018
Collection year: 2018

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
This brief review of the Ventrain ventilation system summarizes the main clinical and technical aspects of the device, with special emphasis on its role in the “Cannot Intubate, Cannot Oxygenate“ situation and in surgery involving the airway. Animal and bench studies characterizing the performance of the device, which is based on Bernoulli's Principle, are also discussed. It is concluded that as clinical experience is accumulated that this new device will play a special role in clinical airway management.
1. INTRODUCTION
Mechanical ventilation of patients is usually carried out using a conventional diameter (e.g. 6 to 8 mm Internal Diameter [ID]) low-resistance cuffed tracheal tube inserted into the patient’s trachea. There are circumstances, however, where this arrangement is clinically unsuitable. For instance, with some forms of laryngeal surgery, a narrow-diameter high-resistance catheter (e.g. Hunsaker catheter) driven by a high-pressure gas source is used instead. This is done in order to offer the surgeon an unimpeded view of the glottic structures. Another situation is the “Cannot intubate, Cannot oxygenate” emergency, where percutaneous Transtracheal Jet Ventilation (TTJV) via a very narrow bore high-resistance catheter is sometimes recommended as a life-saving procedure. Yet another situation involves the use of jet ventilation via a narrow-bore airway-exchange catheter, such as one provided by Cook Medical.
In all these example cases, expiration during ventilation occurs passively. The purpose of this brief review is to provide the reader an overview of the current literature on a system of ventilation that supports active expiration via a narrow-bore catheter. Known as the Ventrain system [1], the device allows for full ventilation via a mere 2 to 3 mm ID catheter, even in fully obstructed airways, using active expiration based on the Bernoulli Principle. The result is a system of ventilation that avoids extreme intrapulmonary pressures and the associated pulmonary (baro)trauma that sometimes marks jet ventilation.
2. PRINCIPLES OF POSITIVE PRESSURE VENTILATION
Positive pressure ventilation has been in clinical use since William Tossach used mouth-to-mouth resuscitation on a coal miner in 1732 [2]. The use of positive-pressure bellows for resuscitation was first recommended in 1776 [3]. Modern ventilators now offer a variety of positive-pressure ventilation modes (Pressure-Controlled Ventilation, Pressure Support Ventilation, Volume-Controlled Ventilation, etc.) with the ability to control ventilation-related parameters such as tidal volume, minute ventilation, respiratory rate, and so on. It is important to note that these ventilators operate using passive expiration ventilation, where the elastic recoil of the chest wall and lungs expel the breath (tidal volume) from the lungs when the applied pressure to the airway is reduced to zero. However, if the applied pressure to the airway is negative, gas is actively suctioned from the lung rather than simply being allowed to exit passively. This is called active expiration, sometimes also known as expiratory ventilation assistance [4, 5]. As discussed herein, the use of active expiration offers a number of important clinical advantages, such as shortening the expiration time when dealing with a high resistance airway, increasing the attainable minute volume and reducing air trapping.
A special ventilation technique is “jet ventilation”, which refers to the delivery of gases (usually oxygen) via high pressure (e.g. 20 to 50 PSI, or about 1000 to 2,500 mm Hg) through a narrow-bore high resistance catheter with passive expiration taking place only through the mouth and nose (providing airway obstruction is not complete). One variant of jet ventilation is High-Frequency Jet-Ventilation (HFJV) a technique involving the delivery of small tidal volumes (typically 1-3 ml/kg) at rates of 1 to 10 Hz [6].
3. BERNOULLI’S PRINCIPLE AND THE VENTRAIN DESIGN
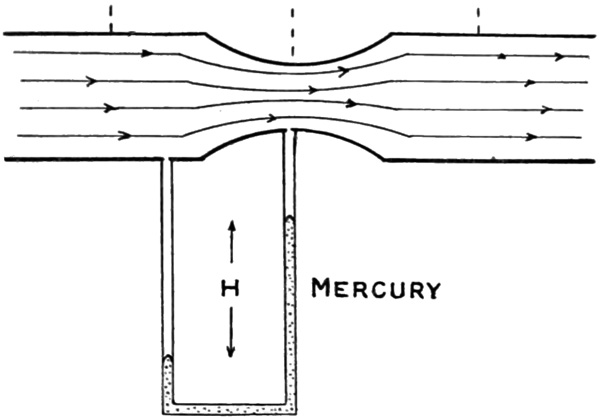
Bernoulli's principle states that an increase in the speed of a fluid (remember that gases such as air and oxygen are both fluids), leads to a decrease in pressure (Fig. 1). This principle is named after Daniel Bernoulli, who described it in his book Hydrodynamica (Fig. 2), published in 1738. It is Bernoulli's principle and its variant, the Venturi effect that forms the basis for the Ventrain system. (The Venturi effect, described in 1797 by Giovanni Venturi, applies Bernoulli's principle to a fluid flowing through a tube with a constriction present).
 |
Fig. (2). Front cover of Daniel Bernoulli’s Hydrodynamica published in 1738. Public domain image courtesy of Wikipedia. |
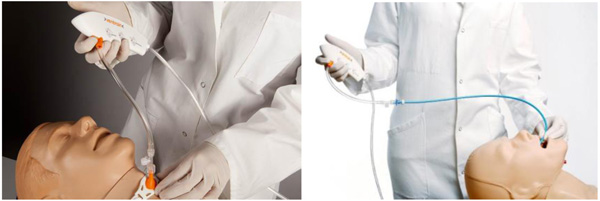
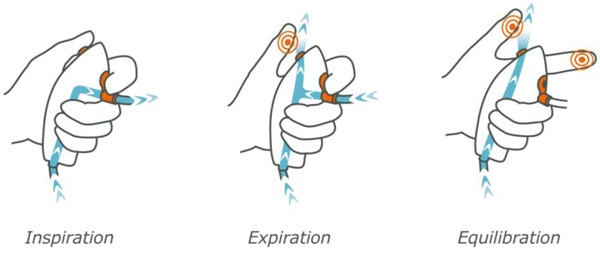
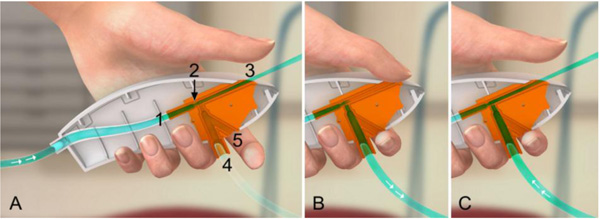
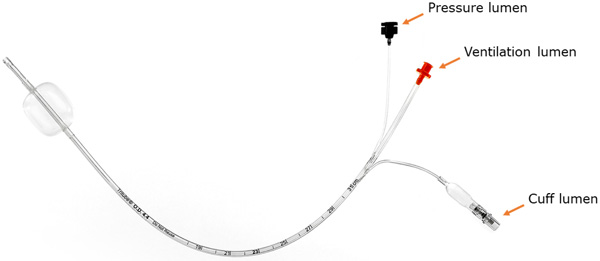
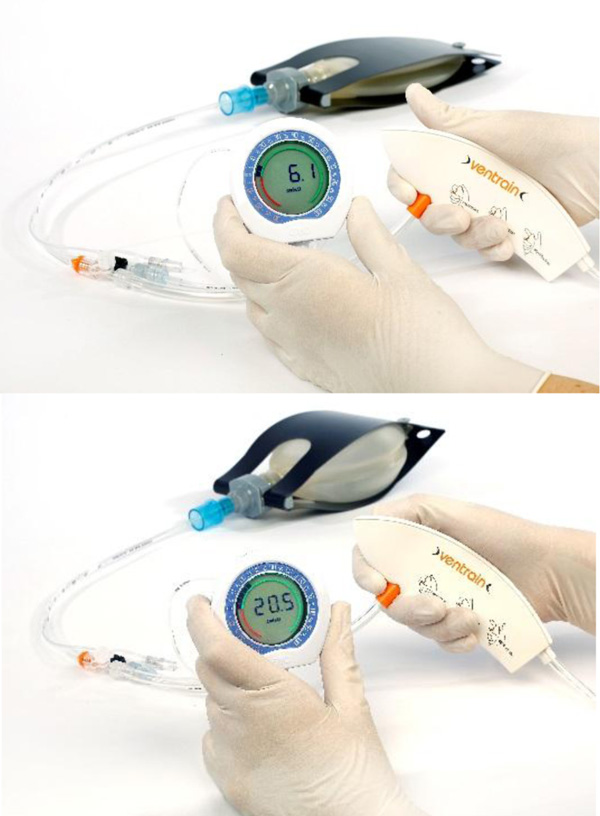
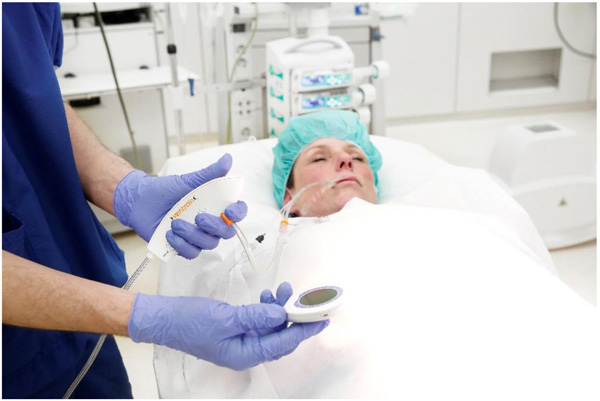
Ventrain (Figs. 3 to 6) is a compact, manually-operated hand-held ventilator specifically designed for ventilation through narrow-bore (e.g. 2 to 3 mm ID) catheters such as the Tritube® narrow-bore cuffed endotracheal tube (Figs. 7 to 9). As noted above, what makes the Ventrain unique in comparison to other ventilation systems is its application of the Bernoulli principle to carry out suction (as opposed to mere passive gas flow) during the expiratory phase of ventilation. This breakthrough in ventilator design allows for minute ventilation rates in excess of 7 liters per minute with relatively modest oxygen flows of 15 L/Min and using catheters with internal diameters as narrow as 2 mm (Hamaekers et al. [1]). Fig. (6) illustrates how Bernoulli's principle is incorporated into the design of the Ventrain.
In bench testing, a pre-release version of Ventrain Hamaekers et al. [1], found that oxygen flows of 6 to 15 litres per minute resulted in driving pressures of 0.5 to 2.3 BAR (7.3 to 33.4 PSI), respectively, and that the maximal suction pressure generated was -217 cm H2O. The maximum gas extraction via suction was 12.4 litres per minute when connected to a 2 mm ID 70 mm long TTC, which was achieved at an oxygen flow of 15 litres per minute, at which “the achievable minute volume through the (test catheter) ranged from 5.9 to 7.1 litres depending on the compliance of the artificial lung.”
4. ANIMAL STUDIES
Numerous animal studies exist demonstrating the functionality of the Ventrain system. Berry et al. [2], studied the Ventrain in comparison to the Manujet in five anesthetized sheep who underwent deliberate desaturation when their proximal airway was completely or critically obstructed. Although both devices allowed for rapid re-oxygenation, there were striking differences in peak airway pressures (16 vs. 40 cm H2O; Ventrain vs. Manujet) and minute ventilation (4.7 vs. 0.1 litres/min, Ventrain vs. Manujet) with the delivered rescue breaths. The authors concluded that the Ventrain system “provided stable oxygenation and effective ventilation at low airway pressure” while with the use of the Manujet system, deliberate hypoventilation was necessary to avoid barotrauma.
Ventrain was for the second time compared with Manujet in a porcine model study conducted by Paxian et al. [3], where 10 pigs were anesthetized and subjected to simulated upper airway conditions (open, partly obstructed airway, or totally obstructed airway). The pigs were then either transtracheally ventilated with the Ventrain system or via manual jet ventilation. The authors found that the minute ventilation as reflected by the arterial CO2 tension “was superior with the Ventrain in partly obstructed airways after 6 min in comparison with traditional manual jet ventilation” as well as in the case of complete airway obstruction. The authors also found that “peak airway pressures were significantly lower and haemodynamic parameters were altered to a lesser extent with the Ventrain” as compared to manual jet ventilation. The authors concluded that their study suggested that “the Ventrain device can ensure sufficient oxygenation and ventilation through a small-bore transtracheal catheter when the airway is open, partly obstructed, or completely closed” and that the Ventrain device provided superior minute ventilation and lower airway pressures in comparison to jet ventilation, particularly in the event of complete upper airway obstruction.
This landmark study was accompanied by an editorial [4] where the author emphasized the potential clinical value of using active expiration via a small-bore cannula, writing that the “working principle behind the Ventrain …(has) the potential to change the way we look at ventilation through small-bore cannulae” both in elective and in emergency situations. However, he also reminded of the journals readership that “as with any other device, the use of the Ventrain requires training and routine use for successful application in emergencies” and that it will always be important “for the user to keep calm and watch the chest movements closely to avoid over-inflation and potential barotrauma.”
De Wolf et al. [5], studied the use of the Ventrain in a porcine model where the airway was obstructed. Instead of a transtracheal catheter a 100 cm long, 3 mm Internal Diameter (ID) Airway-Exchange Catheter (AEC) was used in these six pigs. After desaturation to a SpO2 under 70% took place, Ventrain ventilation through the AEC was started. Within one minute, the saturation increased from a median of 48% before Ventrain ventilation to 100% after. In addition, the PaCO2 was reduced from a median of 59 mmHg to 40 mmHg. The study authors concluded that “in clinical emergency situations of obstructed airways, this device may be able to overcome problems of unintentional hyperinflation and high intrapulmonary pressures when ventilating through long, small-bore catheters and could therefore, minimize the risks of barotrauma and hemodynamic instability.”
Subsequently, De Wolf et al. [6], described their experience using a prototype small-bore ventilation catheter (40 cm long, 2.2 mm inner diameter) with a separate pressure monitoring lumen and with a cuff to seal the airway in combination with the Ventrain. In this study, six anesthetized pigs were used to study ventilation efficiency of Ventrain with the cuff inflated (obstructed airway) and with the cuff deflated (open airway). Much better ventilation and oxygenation were achieved with the cuff inflated, indicating that Ventrain is the most efficient in a sealed/closed airway.
5. CLINICAL USES - UPPER AIRWAY SURGERY
Borg et al. [7], were the first to describe the use of Ventrain during upper airway surgery, chronicling the use of the Ventrain during a laryngoscopy procedure in a stridor patient suffering from an exophytic glottic tumor. A 2 mm ID transtracheal catheter was inserted into the cricothyroid membrane under local anesthesia and connected to the Ventrain using an oxygen flow of 15 liters per minute. General anesthesia was then induced and the patient ventilated via the Ventrain at 15 breaths per minute (inspiration for two seconds, then expiration for two seconds). The procedure, which lasted 15 minutes, was uneventful, and resulted in biopsy samples that allowed for the patient’s condition to be more reliably evaluated.
Kristensen et al. [8], used the Ventrain in conjunction with a special 2.4 mm ID tracheal tube (Tritube®) in seven ENT surgical patients. The authors noted that this arrangement provided an “unprecedented view of the intubated airway during oral, pharyngeal, laryngeal or tracheal procedures” and even noted that the technique has the “potential to replace temporary tracheostomy, jet-ventilation or extra-corporeal membrane oxygenation in selected patients.” In this series, the authors also mention that one patient was transported to the PACU with a Tritube in place (with the cuff deflated) and was subsequently uneventfully extubated one hour after surgery.
Onwochei et al. [9], described a two-stage procedure to manage airway obstruction and avoid a surgical tracheostomy in a 49-year-old woman who suffered from “postradiotherapy laryngeal fixation and transglottic stenosis” and had a pharyngeal stricture in need of dilation. Their technique involved awake fiberoptic intubation, followed by the transtracheal insertion of a Cricath flexible 2-mm needle cricothyrotomy catheter, with ventilation using the Ventrain system. Similarly, Fearnley et al. [10], described the elective use of the Ventrain for upper airway obstruction in a patient with post-radiation fibrosis that had previously prevented passive expiration during attempting high-frequency jet ventilation.
6. CLINICAL USES – “CANNOT INTUBATE, CANNOT OXYGENATE” SITUATION
Needle cricothyroidotomy in conjunction with Transtracheal Jet Ventilation (TTJV) is sometimes advocated as a last-ditch means options to avoid death in a ‘Cannot intubate, Cannot oxygenate” (CICO) situation [11-13]. In TTJV, oxygen is injected percutaneously under high pressure (e.g. 20 PSI) via a narrow-bore, high-resistance cannula, typically using a 12 or 14-gauge intravenous catheter or a purpose-built catheter such as that make by Cook Medical (Fig. 10).
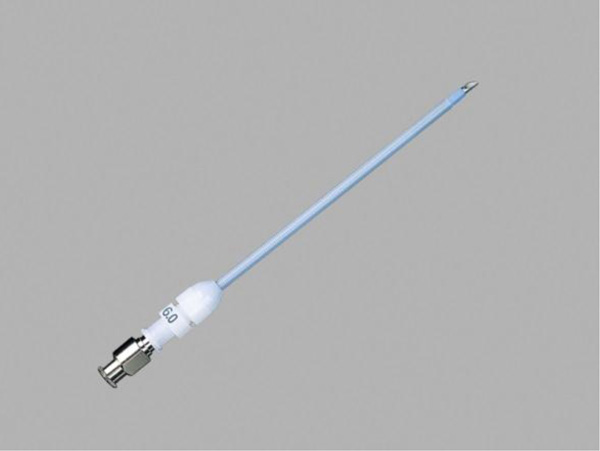 |
Fig. (10). The Cook Medical Emergency Transtracheal Airway Catheter is designed for use in emergency TTJV. The 6 Fr diameter device is wire-reinforced to prevent kinking, a problem that has been identified when ordinary intravenous catheters are used. It is available in 5 and 7.5 cm lengths. Image Credit: https://www.cookmedical.com/products/cc_dtjvbtt_webds/. |
Unfortunately, this technique of ventilation is associated with a number of safety concerns in that expiration of the injected gas in this setting must take place via the nose and/or mouth since due to its very high resistance passive gas expiration via the transtracheal catheter negligible. As a result, in situations where complete airway obstruction exists and no pathway for gas egress exists, repeated injections of oxygen will cause pressure build up that may produce barotrauma events such as pneumothorax or subcutaneous emphysema, events that in turn lead to hemodynamic deterioration or even full cardiac arrest. In a meta-analysis by Duggan et al. [14], forty-four studies involving 428 TTJV procedures were reviewed. The authors found that device failure occurred in 42% of CICO emergencies, with barotrauma occurring in 32% of CICO emergencies. In some cases TTJV-related subcutaneous emphysema made subsequent attempts at airway management much more difficult. The authors concluded that “TTJV is associated with a high risk of device failure and barotrauma in the CICO emergency” setting and that “recommendations supporting the use of TTJV in CICO should be reconsidered.”
Duggan et al. are not alone in being concerned that TTJV via cannula cricothyroidotomy is potentially perilous. The NAP4 study1 offers the following commentary (p. 9):
There was a high failure rate of emergency cannula cricothyroidotomy, approximately 60%. There were numerous mechanisms of failure and the root cause was not determined; equipment, training, insertion technique and ventilation technique all led to failure. In contrast, a surgical technique for emergency surgical airway was almost universally successful. The technique of cannula cricothyroidotomy needs to be taught and performed to the highest standards to maximise the chances of success, but the possibility that it is intrinsically inferior to a surgical technique should also be considered. Anaesthetists should be trained to perform a surgical airway.
The results of the NAP4 study and the experience of others have lead many experts to recommend the abandonment of cannula cricothyroidotomy methods in favour of using a surgical approach. The Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults [15] note that “scalpel cricothyroidotomy is the fastest and most reliable method of securing the airway in the emergency setting” and emphasize that “whilst a wire-guided technique may be a reasonable alternative for anaesthetists who are experienced with this method, the evidence suggests that a surgical cricothyroidotomy is both faster and more reliable.”
A study by Heymans et al. [16], tested the performance of medical students previously untrained in surgical airway techniques in carrying out a cadaveric cricothyroidotomy by three different techniques: surgical technique, use of the Melker kit, and use of the QuickTrach II kit. The success rates were 95%, 50% and 55% respectively. The authors concluded that in surgical airway-naive medical personnel emergency cricothyroidotomy will be achieved “more efficiently and safely with the surgical procedure” than with the other techniques studied. An accompanying editorial by Asai [17] concluded that “the current state of knowledge indicates that surgical cricothyroidotomy is more reliable than percutaneous cricothyroidotomy as a rescue method in the “Cannot intubate, Cannot oxygenate” situation. It is now time for us to establish a standardized airway crisis management system and to undergo training on a regular basis to rescue our patients from hypoxic damage.”
In a similar vein, Schulze et al. [18], investigated the performance and acceptance of a proposed airway management algorithm and training program for pulmonologists providing propofol sedation. The algorithm includes using 3 maneuvers, including bag mask ventilation, use of a laryngeal tube type supraglottic airway, and use of a needle cricothyrotomy using a Cricath 2-mm needle cricothyrotomy catheter in conjunction with the Ventrain. The authors concluded that their program “may be a valuable tool for ensuring patient safety” during propofol sedation administered by nonanesthesiologists.
Given the identified concerns using TTJV in conjunction with a high-pressure source, the Ventrain system may end up having a preferred role as a rescue device in the “Cannot Intubate, Cannot Oxygenate” (CICO) situation since it would lower the frequency of events which demand percutaneous airway access and it allows smaller diameter catheters to be used for surgical cricothyroidotomies and other airway interventions, as illustrated by the following case report and similar cases. Such reports suggested that some cases of CICO would not be so if the patient had been “intubated” with an AEC and ventilated with the Ventrain device.
Wahlen et al. [19] described the use of the Ventrain system in a 77-year-old tracheotomised woman who developed life-threatening tracheal stenosis. After attempts at intubation with a 5.0 mm ETT failed, the patient was ventilated via a tube exchanger using the Ventrain system until a new tracheostomy could be completed.
7. CLINICAL USES - PEDIATRICS
Willemsen et al. [20] have provided two paediatric case reports on the emergency management of critical paediatric airways using the Ventrain. In the first case a 2.1 kg baby developed delayed but life-threatening airway loss (arterial saturations below 40%) following repeated unsuccessful attempts at tracheal intubation. A subsequent attempt at direct laryngoscopy revealed glottic edema that was so severe that a 2.5 mm ID tracheal tube could not be positioned. The child’s airway was rescued by advancing a Frova intubating catheter (ID 1.66 mm) into the trachea and connecting this to the Ventrain.
In a second case involving a larger baby (4.3 kg) similar difficulties were encountered in advancing a tracheal tube into the glottic aperture (first a 3.0 mm ID tube, then a 2.5 mm ID tube) but the airway was ultimately secured by first advancing a Cook tracheal tube exchange catheter (ID 1.66 mm), ventilating the child using the Ventrain, and then advancing a 3.0 mm ID uncuffed tube over the catheter with videolaryngocopy assistance.
The authors noted that “use of the Ventrain in combination with small lumen intubating or tube exchange catheters is a new alternative in paediatric airway emergencies” but caution clinicians that the absence of intrathoracic/intrapulmonary pressure monitoring in this setting mandates that clinicians “closely observe chest movements and to adjust the I:E ratio properly to avoid lung injury.”
Escribá Alepuz et al. [21] describe emergency ventilation of an infant with subglottic stenosis using the Ventrain. When the patient desaturated, the device provided immediate rescue ventilation during rigid bronchoscopy with restoration of normal oxygen saturation. Then, post intubation, ventilation with the Ventrain was again valuable, when conventional mechanical PICU ventilation became very difficult due to elevated pressures. This case clearly indicates that the new ventilation device Ventrain offers advantages over devices available until now.
Finally, note that while many experts advocate the “scalpel bougie” approach over other methods in establishing an emergency surgical airway in adults, the situation is more complicated in the pediatric population. Anatomically, a child's neck is different from that of an adult in several ways: the neck is shorter (meaning less working space), the more elastic paediatric trachea can be more difficult to palpate, the cricoid cartilage may be difficult to identify, and the dome of the pleura extends into the neck, making it susceptible to accidental surgical injury. In addition, the pediatric cricothyroid membrane is quite narrow. Such considerations have led experts to recommend against cricothyroidotomy surgery in children less than 12 years of age, as it offers greatly increased risk of permanent laryngeal injury [22]. These considerations have also led some investigators to explore the best approach to establishing emergency tracheostomies in children.
As an example, Prunty et al. [23] compared Cook’s Melker cricothyroidotomy kit with the “scalpel bougie” technique for tracheotomy surgery on a cadaveric rabbit 'infant airway' model. The Melker technique achieved a 100% success compared to a 75% success rate for the “scalpel bougie” technique. The authors noted that the first attempt success rate using the Melker kit “was dependent on the level of the tracheotomy (Level 1 100%, level 2 62.5% and level 3 & 4 25%)” and found that both methods (but especially the “scalpel bougie” technique) led to frequent lateral and/or posterior tracheal wall damage. Of course, as with many other situations, prevention is preferred over cure, so in case of an anticipated lost or difficult pediatric airway, one may consider preventing airway loss by placing a pediatric exchange catheter, when possible.
8. CLINICAL USES - LUNG ISOLATION
Lung isolation is a frequent requirement in thoracic surgery, and can be achieved by a number of methods [24]. The usual goal of lung isolation during thoracic surgery is to provide needed exposure for the surgeon by deflating one of the lungs while ventilating the other. However, one-lung ventilation greatly increases the risk of hypoxemia to the extent that oxygenation of the deflated is sometimes required. While this is often achieved using Continuous Positive Airway Pressure (CPAP), this is not always practical. Consequently, some clinicians are advocating use of the Ventrain system in some special thoracic cases.
As an example, Evers et al. [25] described a patient who, while undergoing a thoracoscopic esophagectomy with concomitant wedge resection, developed a tear in the left main bronchus. Unfortunately, because the bronchial cuff of the double-lumen tube was protruding through the tear, repair was only possible after bronchial cuff deflation. The situation was cleverly resolved using the Ventrain system to ventilate the patient through a 65-cm 7F Arndt
2 Chapter 48, pages 44-87, Hagberg and Benumof’s Airway Management, 2017, ISBN: 9780323428811)
Endobronchial Blocker placed through the bronchial lumen beyond the bronchial defect. Alternatives that might also have been tried include using a catheter suitable for high-frequency jet ventilation or instituting cardiopulmonary bypass.
9. CLINICAL USES - EXTUBATION
In a comprehensive chapter on extubation and reintubation of the difficult airway2, Cooper discusses the various devices that can be used to make extubation safer in high-risk patients, such as airway exchange catheters, endotracheal ventilation catheters and jet stylets. He notes that use of some of these devices have resulted in patient injury as a result of barotrauma, and suggests that oxygen insufflation at low flows (1-2 liters per minute) is at times a more appropriate choice than high-pressure jet ventilation. However, he also suggests that the Ventrain device may offer an even better solution in avoiding barotrauma as it “appears to produce active flow-dependent inspiration and expiration … with much less risk of barotrauma.”
CONCLUSION
The Ventrain device offers a new way of patient ventilation based on the Bernoulli Principle. It is quite likely that as clinical experience is accumulated that this new device will play an increasingly important role in clinical airway management, especially in (prevention of) the rescue of a lost airways and in complex airway surgery. In addition, use of the Ventrain in elective settings such as upper airway surgery also helps physicians develop confidence in using the Ventrain in a stressful CICO settings.
NOTES
2 Chapter 48, pages 44-87, Hagberg and Benumof’s Airway Management, 2017, ISBN: 9780323428811)
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.