All published articles of this journal are available on ScienceDirect.
Dexmedetomidine in the Management of Awake Fiberoptic Intubation
Abstract
Awake Fibreoptic Intubation (AFOI) is, nowadays, the gold standard in predicted difficult airway management. Numerous practice guidelines have been developed to assist clinicians facing with a difficult airway. If conducted without sedation, it is common that this procedure may lead to high patient discomfort and severe hemodynamic responses. Sedation is frequently used to make the process more tolerable to patients even if it is not always easy to strike a balance between patient comfort, safety, co-operation, and good intubating conditions. In the last years, many drugs and drug combinations have been described. This minireview aims to discuss the evidence supporting the use of Dexmedetomidine (DEX) in the AFOI management.
1. INTRODUCTION
The estimated incidence of patients with difficult airway during clinical anesthesia is 1-18%; in these patients with a difficult airway, the anatomy is frequently different from normal, and the inadequate airways management may result in hypoxemia, hypoventilation, aspiration, brain damage or even death [1].
Up to 30% of all deaths attributable to anesthesia are related to difficult airway management [2].
Awake Fiberoptic Intubation (AFOI) is considered the Gold Standard in patients with a predicted difficult airway [3].
This methodology could be conducted in totally awake patients after airway local anesthesia, under conscious sedation or by combining both approaches to limit airways reactivity. During AFOI, coughing and laryngospasm in reaction to intubation can be problematic, so an effective airway local anesthesia is mandatory for the comfort of the patient and subsequent success of the procedure. Proper local anesthesia also seems to reduce sedation doses of midazolam and fentanyl [4, 5].
When AFOI is effectuated without sedation, it is commonly related to patient discomfort and severe hemodynamic responses, inducing catecholamine release by sympathetic stimulation, sympathetic stimulation, which may result in increased heart rate and blood pressure, arrhythmia, and cause myocardial ischemia and infarction in patients with risk factors, such as hypertension and ischemic heart disease [6, 7]. The sedation may alleviate the awake intubation, but it requires conscientious administration and continuous monitoring as it can lead to airway obstruction and hypoxemia, while inadequate sedation could lead to discomfort, anxiety and excessive sympathetic discharge.
2. CONSCIOUS SEDATION IN AFOI MANAGEMENT
Different pharmacological approaches have been reported to obtain conscious sedation and prevent cardiovascular changes during AFOI such as local anesthetics, benzodiazepines, opioids, α2 adrenoceptor agonists and less commonly propofol or ketamine [5, 8-14]. In order to achieve the ideal conditions for AFOI, the patient should be comfortable, compliant, does not present excessive oropharyngeal secretion or blood, and with the ability to maintain spontaneous ventilation to tolerate the passage of a fiberscope in order to facilitate fiber-optic intubation [15]. It is remarkable for patients undergoing sedated but awake AFOI to have decreased anxiety, discomfort, and hemodynamic disturbances.
The ideal drug to obtain these conditions should, therefore, be short-acting and easily titratable to obtain an adequate sedation level, with minimal effects on spontaneous ventilation [16, 17]. Anyhow, it is commonly arduous to provide these conditions using a single medication or approach. In Table 1, a synthesis of the effects or the more commonly used drugs to manage AFOI is presented.
3. AFOI WITH OPIOID-BASED SEDATION
Commonly, opioids combined with benzodiazepines are utilized for sedation in AFOI. It has been described that sufentanil, alfentanil, fentanyl, and other opioids minimize the alteration elicited by fiberoptic intubation [18-21]. However, opioids have the risk to induce respiratory depression. Sufentanil has been compared with fentanyl, and it has shown to obtain deeper and longer analgesia, presently shorter episodes of respiratory depression, but if used alone for AFOI, it can induce a greater incidence of recall [22, 23].
In a recent study, Tsukamoto et al. describe the cases of sedated AFOI using small doses of midazolam and fentanyl in combination with effective local lidocaine airway anesthesia, reporting good results on the success of the procedure with minimal hemodynamic changes and with minimal discomfort for the patient [24].
Remifentanil is now under several investigations stimulating great interest especially because it has both analgesic and antitussive properties during awake intubation while allowing communication with the patient to be maintained. The use of remifentanil has been described to be helpful for awake intubation with or without the combination of local airways anesthesia [25-28]. Some studies have investigated the optimal dose of remifentanil required for sedation by TCI during AFOI.
Rai and collaborators suggested that a target concentration of remifentanil of 3.2 (2.8-3.5) ng/ml provided adequate sedation for AFOI. This study shows a median endoscopy score different than that of similar studies with a similar concentration of remifentanil, probably for premedication with midazolam and for reaching the deeper local airways anesthesia [11-13].
In this study the authors described how, in remifentanil groups without midazolam premedication, there has been greater recall of intubation, this may indicate that remifentanil has less of an amnesic effect than DEX at the same level of sedation. According to the author DEX and remifentanil were both effective in patients undergoing AFOI but DEX offers better endoscopy scores, lower recall of intubation, and greater patient satisfaction, with minor hemodynamic side effects.
Moreover, these results should also be complemented by the evidence reported by Vennila and colleagues asserting that intubation was achieved with a higher remifentanil concentration without the use of local anesthesia and benzodiazepine [11].
Some singular characteristics of DEX make it a useful solution for sedation in AFOI [5]. In addition to hemodynamic stability, anxiolytic and analgesic properties can also determine a deep state of sedation while maintaining easy arousability without causing respiratory depression, even at higher doses. Moreover, it also decreases salivary secretions, allowing better visualization through the fiberscope and decreasing the risks of inhalation.
4. USE OF DEXMEDETOMIDINE IN AFOI
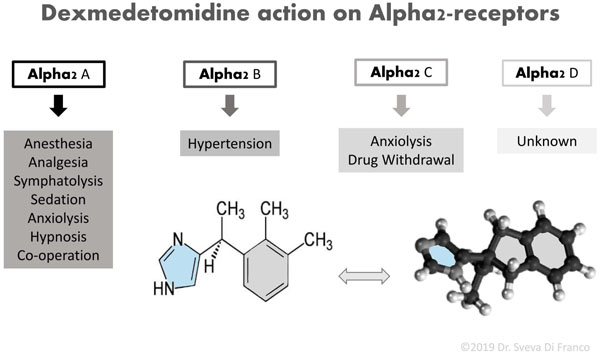
Dexmedetomidine is an alpha2-adrenergic agonist (C13 H16HCl), comparable for its structure to clonidine but with a greater affinity, 8 to 10 times more selective, for alpha2-receptors over alpha1-receptors [29].
It is the dextrorotatory S-enatiomer of medetomidine chemically described as (+)-4-(2,3-dimethyle phenyl) ethyl-1H -imidazole monohydrochloride.
Recently it has been introduced to anaesthesia practice.
Fig. (1) reports a scheme of DEX chemical structure and summarizes the action of DEX on all subtypes of alpha2-adrenergic G-Protein-coupled receptors.
The action on postsynaptic alpha2-adrenergic receptors in the locus coeruleus explains the sedative and anxiolytic effects of DEX, decreasing the sympathetic tone. Therefore, the DEX can influence the release of substance P in the dorsal horn of the spinal cord providing its analgesic effects [30]. It has to be noticed that these subtypes of receptors are found ubiquitously in the central, peripheral, and autonomic nervous systems, as well as in vital organs and blood vessels [31]. Chrysostomou et al. described that the administration of DEX alone gives analgesia and sedation but shows benzodiazepine- and opioid-sparing effect when used with anesthetic drugs [32] DEX is of interest because it possesses sedative, anxiolytic, analgesic and sympatholytic properties during awake intubation, without affecting the respiratory function [33]. Furthermore, DEX is characterized by a rapid distribution phase with a distribution half-life of six minutes and a terminal elimination half-life (t1/2) of two hours. It exhibits linear kinetics between 0.2 - 0.7 micrograms (mcg)/kg/hr on i.v. Infusion up to 24h [34].
| Drugs Used in AFOI and their Effects | |||
|---|---|---|---|
| Drugs Effects | Oppioids | Benzodiazepines | Dexmedetomidine |
| Respiratory depression | ++ | + | - |
| Reduction in pain and discomfort | ++ | ++ | ++ |
| Analgesy | ++ | + | ++ |
| Sedation | ++ | ++ | ++ |
| Antitussive effect | ++ | ++ | ++ |
| Patients consciousness | + | + | ++ |
| Hemodynamic impact | ++ | + | - |
Some pieces of evidence seem to reveal that DEX reduces the sympathetic nervous system response, and inhibits harmful cardiovascular changes by reducing the noradrenaline release [35]. Furthermore, it may induce, at high dosage, side effects including hypotension, bradycardia, atrial arrhythmia, and hypoxia [36].The efficacy of DEX as a sedative agent in the AFOI has been described with encouraging results in several studies [16, 22, 37-41]. Moreover, some authors described how DEX exhibits better sedation than propofol, fentanyl and remifentanil during AFOI [37, 38]. In a systematic review, He et al. have shown that DEX reduces participants’ discomfort with no significant differences in airway obstruction, low oxygen levels or management of cardiovascular adverse events noted during AFOI compared with control groups [42]. In a randomized double-blind study, the authors compare the effectiveness of sufentanil with midazolam and DEX with midazolam for conscious sedation for AFOI in combination with topical airway anesthesia with a “spray-as-you-go” method. In this study, the results were found to be comparable for the two groups in terms of patients’ tolerance and intubating conditions without affecting the patency of the upper airway. However, it has proved to be important to keep patients of sufentanil group responsive and compliant for the potential risk of respiratory depression [43]. Compared with placebo, in a multicenter study, Bergese et al. evaluated the safety and efficacy of DEX for Sedation During AFOI, showing that considerably less DEX patients than placebo patients needed midazolam to obtain and sustain the required sedation. The dose of midazolam required for sedation was significantly lower in the DEX group than in the placebo group, without compromising spontaneous ventilation [41].
In 2013, double-blinded randomized controlled trial, Hu et al. compared the DEX with remifentanil for sedation during AFOI [40]. This study described that both drugs were effective in patients undergoing AFOI. Although fibroscopy was more tolerated with DEX, there were no substantial differences in intubation scores in the two groups. However, DEX group showed better endoscopy score, lower recall of intubation, and greater patient satisfaction, with minor hemodynamic side effects. The analgesic effect of DEX plays an important role in improving patient comfort during AFOI, giving better intubation scores. Moreover, the antitussive effect of remifentanil minimizes the incidence of coughing during fibroscopy. This effect may be responsible for intubation scores comparable between the two groups, against higher endoscopy scores in the remifentanil group. DEX induces conscious sedation by activating the endogenous sleep-promoting pathway. Patients sedated with DEX are awakened more easily when stimulated than those sedated with midazolam; in addition, they experience little respiratory depression [39-44]. Furthermore, xerostomia described as an effect of DEX can provide a better field for fibroscopy by increasing visibility and reducing the risk of inhalation [45, 46]. The optimal dose of DEX required to achieve conscious sedation has been investigated in several trials [41].
However, it has been shown that a loading dose of 1 mcg/kg followed by a 0.7 mcg/kg/h maintenance infusion of DEX is able to induce satisfactory sedation level in patients undergoing AFOI with or without low auxiliary doses of midazolam [47].
CONCLUSION
Dexmedetomidine for it sedative, anxiolytic, analgesic and sympatholytic properties may be considered as a useful drug during awake intubation, reducing participants’ discomfort, without depressing respiratory function and having a negligible impact on the cardiovascular system.
The findings of this review should be corroborated by additional investigations.
Additional investigations should corroborate the findings of this review.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


