All published articles of this journal are available on ScienceDirect.
Acoustical Respiratory Monitoring in the Time Domain
Abstract
This paper introduces the reader to some of the various methods that are available for the time-domain bio-acoustical monitoring of patient breathing. Technical details concerning microphone selection, calibration and characterization, signal amplification, signal filtering and waveform recording are presented. We also describe proof of concept recordings obtained from the neck, from the external ear canal, from a microphone embedded into an oxygen mask and from a leak-free microphone pneumatically connected to the cuff of a laryngeal mask airway. We recommend Audacity, an open-source digital audio editor and recording package that can be freely downloaded at https://www.audacityteam.org for investigators seeking to conduct research on breath sound analysis.
1. INTRODUCTION
In contemporary medicine the need for respiratory monitoring has become especially important with the heavy use of opioids for perioperative pain management [1-3]. Despite this clinical need, no simple dependable method of continuous respiratory monitoring has come into routine clinical use, although the Masimo system of respiratory monitoring [4] and capnography (CO2 monitoring) come close. Capnography is amongst the most popular method of continuous respiratory monitoring but suffers from a need to continually ensure that the gas sampling system is operating correctly [5]. Extraction of respiratory information from the pulse oximeter photoplethysmograph signal remains a field of active research [6, 7] but has not yet come into the mainstream. A device known as the ExSpiron 1Xi Monitor Pack (Respiratory Motion Inc., Waltham, MA) [8-10] operates by detecting electrical impedance changes in the thorax and respiratory muscles and seems to be particularly promising, although it is neither simple nor inexpensive.
In addition to reviewing some of the practical fundamentals of practical acoustical respiratory monitoring this report provides information of potential value to investigators who wish to develop up their own system for digital recording and analysis of breath sounds with a view to exploring how acoustical methods of respiratory monitoring might be improved. Preliminary results are provided in this report on the potential value of studying breath sounds recorded from various locations: from the neck, from the external ear canal, from a microphone embedded into an oxygen mask and from a leak-free microphone pneumatically connected to the cuff of a laryngeal mask airway.
1.1. Breath Sounds
Breath sounds are the result of laminar and turbulent gas flow in airway structures [11, 12]. Normal breath sounds are often classified as tracheal, bronchial, bronchovesicular, and vesicular sounds, depending on where the auscultation is carried out. For example, tracheal breath sounds obtained over the trachea are sometimes described as harsh, like the sound of air is being blown through a pipe, while vesicular sounds are sometimes described as “soft, blowing, or rustling sounds” normally found throughout inspiration, and continuing, fading about one third of the way through expiration.i In addition to descriptions based on their location, normal breath sounds are often described by their duration, their intensity, their pitch (frequency content) and their timing (e.g., inspiratory vs expiratory) within the respiratory cycle.
Pathological changes sometimes change the characteristics of lung sounds. Under such conditions, the regular lung sounds may contain additional superimposed pathological sounds, known as adventitious sounds. If they are mostly continuous, they are called “wheezing” when high pitched, and “rhonchi” when low pitched. Wheezing is often found in asthmatic patients suffering from bronchospasm. Rhonchi are often found heard in patients with Chronic Obstructive Pulmonary Disease (COPD), bronchiectasis, pneumonia, chronic bronchitis, and cystic fibrosis.
If the adventitious sounds tend to be discontinuous and “explosive” in character they are often known as fine crackles (or rales, to use the old term) when high pitched, and as coarse crackles (or rales) when low pitched. Crackles are often found in a lung field that has fluid in the small airways and are thought to be due to the sudden reopening of the small airways previously closed by surface forces [11]. The characteristics of crackles sometimes offer helpful diagnostic information [13]. For example, crackles that do not clear after a cough are suggestive of pulmonary edema or pulmonary fibrosis (and several other conditions) while crackles that clear or change after coughing are more commonly found (for example) in bronchiectasisii.
1.2. Apnea Detection
Liu et al. [14] recorded tracheal sounds from 121 Post-Anesthesia Care Unit (PACU) patients using a microphone encased in a plastic bell along with a processed nasal pressure signal used as a reference method. The logarithm of the tracheal sound variance was used for apnea detection. Their new algorithm detected apneas with 92% sensitivity and 98% specificity compared to the reference method.
Other applications of tracheal sound analysis include detecting episodes of sleep apnea [15], and apneic episodes during procedural sedation [16]. MacGregor et al. [17] conducted a statistical analysis of tracheal breath sounds during wakefulness with a view to screening for obstructive sleep apnea.
2. THE RESPIRATORY CYCLE
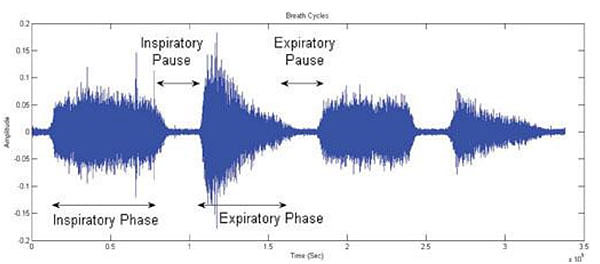
The respiratory cycle is typically described as consisting of four phases that repeat endlessly (Fig. 1)
(1) Inspiratory Phase: From the moment that air is drawn into the lungs to the moment that inspiratory airflow stops.
(2) Inspiratory Pause: From the moment that inspiratory airflow stops to the beginning of the expiratory phase.
(3) Expiratory Phase: From the moment that air exits the lungs to the moment that expiratory airflow stops.
(4) Expiratory Pause: From the moment that expiratory airflow stops to the beginning of the next inspiratory phase.
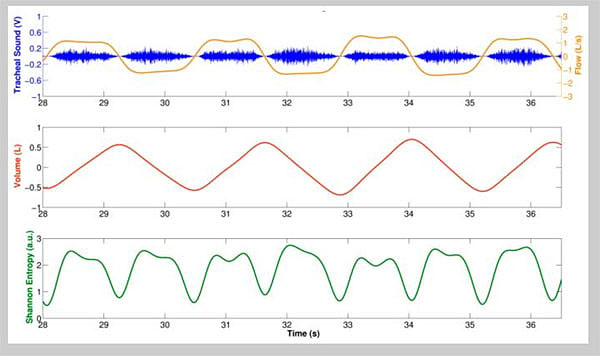
One of the challenges in a number of clinical monitoring settings is to accurately identify the above respiratory phases. For example, in some studies of blood pressure variability and heart-rate variation blood pressure wave and ECG recordings are separated into inspiratory and expiratory phases to allow documentation of cardio-respiratory interactions. Although this can be achieved relatively easily using spirometry or impedance recordings, the prospect of automatically separating lung sound signals into inspiratory and expiratory components without additional instrumentation has also met with success, for example using Shannon Entropy of respiratory sound data (Fig. 2).
2.1. Using Microphones to Indirectly Listen to Breath Sounds
Both precordial stethoscopes and esophageal stethoscopes can be to directly listen to heart sounds as well as breath sounds. In addition to using purely acoustic devices, monitoring of bioacoustical phenomena can also be achieved using electronic means, incorporating a microphone and amplifier. Both wired and wireless commercial products are available, as well as units based on Bluetooth technology [18, 19]. Electronic stethoscopes are available from companies such as Cardionics (cardionics.com), Ekuore (www.ekuore.com) and ThinkLabs (https://www.thinklabs.com/), although some of these products focus on cardiac applications. ThinkLabs offers a library of recorded breath sounds (lung sounds) at https://www.thinklabs.com/lung-sounds that many clinicians may find to be useful.
ii For a sampling of normal and pathological breath sounds, the interested reader is directed to https://soundcloud.com/sam-alsmadi, http://www.cvmbs.colostate.edu/clinsci/callan/breath_sounds.htm and https://www.thinklabs.com/lung-sounds
Investigators seeking to setup a research program involving bio-acoustical phenomena often employ a miniature electret microphone that is easily connected to stethoscope heads or other equipment. Much of the early work at our laboratory utilized a RadioShack 33-3013 miniature omnidirectional electret microphone which was secured to a stethoscope head (or other device) under test via a short piece of tubing and then amplified using an ordinary audio amplifier such as a RadioShack miniature audio amplifier (Catalog # 2771008). While this arrangement works well, the recent commercial availability of USB-connected miniature electret microphone has made it even easier to obtain quality bio-acoustical recordingsiii. This is because USB microphones require no built-in battery (it obtains its power instead from the USB connection) and contains a built-in amplifier as well. An additional advantage of USB type microphones is that the final signal obtained does not depend on the characteristics of the analog and digital circuitry within the host computer. One potential disadvantage, however, is the lack of a gain adjustment control in the built-in amplifier in most USB type microphones.
2.2. Recording Sites for Breath Sounds
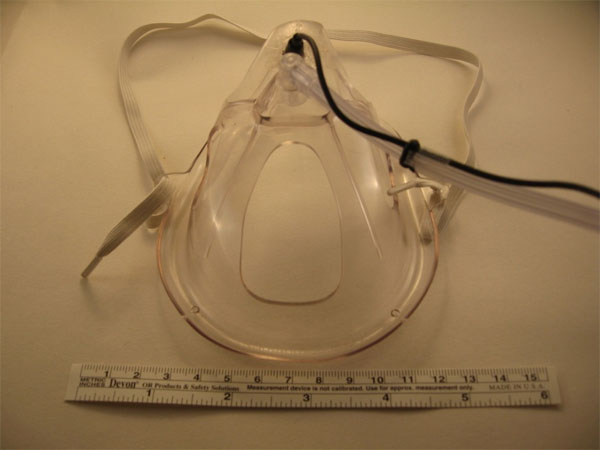
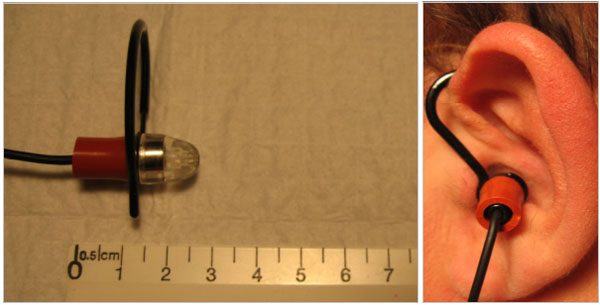
There exist several locations from which breath sounds can be recorded, such as at the neck (Fig. 3), over the trachea, and at the anterior or posterior chest walls. In addition to these conventional sites, we have also obtained quality breath sound recordings embedding a miniature electret microphone into an oxygen mask (Fig. 4) as well as sounds of somewhat lower quality by placing a modified electret microphone assembly into the external ear canal (Fig. 5). Finally, we have shown that it is even possible to record breath sounds from a supraglottic airway [20]. (For a sample recording of breath sounds (and other sounds) obtained from a supraglottic airway placed in a surgical patient undergoing general anesthesia visit http:// lmamonitor.homestead.com/).

iii. For example, see https://www.amazon.com/Microphone-ZAFFIRO-Lavalier-Recording-Interviews/dp/B0747F2HHG
3. MICROPHONE CALIBRATION
In research settings it can sometimes be necessary to establish the output characteristics of a microphone when it is subjected to a known acoustic signal iv. For small microphones this can be most easily achieved by inserting the microphone into a calibrator such as the unit shown in Fig. (6) and measuring the microphone output using an oscilloscope or by other means. The unit in Fig. (6) provides a 94 dB ± 0.5 dB (SPL) reference acoustic reference signal at 1000 Hz.
For larger microphones, an alternative calibration method is “free field calibration” whereby a 1000 Hz tone (for example) is fed to an amplifier/loudspeaker assembly to generate an acoustic signal filling the room. The signal level at a particular point in the room is then measured using a Sound Pressure Level meter, such as the unit shown in Fig. (7). The microphone under test is then placed beside the Sound Pressure Level meter that is measuring the intensity of the acoustic reference signal and the output of the microphone is then measured and calibrated against the reading of the Sound Pressure Level meter.


4. ELECTRONICALLY RECORDING BREATH SOUNDS USING A HAND-HELD RECORDER
High-fidelity hand-held audio recording systems are available that are quite suitable for the digital recording of bio-acoustical signals. Typically, these support two or more audio channels and record the data in either the WAV or MP3 audio formats (vide infra). Automatic gain control is a common feature in such products, but this feature may need to be disabled when calibrated recordings are desired. As an example, the battery-operated TASCAM DR-40 Digital Recorder used in our laboratory offers both built-in microphones as well as support for external microphone inputs in XLR and TRS format. The unit also allows for recordings to an SD memory card to be made in both WAV and MP3 recording formats.
iv For detailed technical discussion on the subject of microphone calibration visit http://www.pcb.com/Contentstore/mktgcontent/WhitePapers/WPL_36_Acoustic_methods_calibration_PCB.pdf
5. ELECTRONICALLY RECORDING BREATH SOUNDS USING A SMARTPHONE
Smartphones can be used to record breath sounds using an external microphone in two ways. The first way to connect an external microphone to the input jack of the smartphone normally used to attach a headset. The headset jack of the smart phone typically has a ground and three connections, two connections being for the left and right headphone channels and the remaining connection being a microphone input. To use this approach for a custom design it is necessary to begin by searching for information pertaining to the pinout of the headset jack of the smart phone in question as well as determine the required characteristics of any attached microphone (e.g., some microphones require a 1.5 or 2-volt power source). While this approach has promise, it can require considerable effort as the required information is not always readily available.
A second more practical approach is to purchase an external microphone specifically designed for use with a particular smartphone, such as the iPhone. Inexpensive examples from amazon.com are https://www.amazon.com/dp/ B074KJ1N3D (single microphone unit) and https://www. amazon.com/dp/B071X6H6ZS (dual microphone unit).
A third approach is to use a Bluetooth microphone, but these are hard to find in the small size needed for bio-acoustics work.
As noted earlier, a final approach is to use a USB lavalier microphone, or a USB audio mixer connected to an Android smartphone via an OTG adapterv. The USB approach has the important advantage that recalibration is not needed when switching from one computer or smartphone to another, as the data is digitized in the microphone unit itself rather than in the computer or smartphone.
Reyes et al. [21] described their experience in acquiring tracheal sounds using the Samsung Galaxy S4 and the iPhone 4s smartphones to acquire tracheal sounds from nine healthy volunteers at airflows from 0.5 to 2.5 L/s measured using a spirometer. Tracheal sounds were acquired at the neck using a miniature electret microphone encased in a plastic bell and recorded using the built-in audio recorder application of each smartphone (Voice Recorder in the Galaxy S4, and Voice Memos in the iPhone 4s). These recordings were transferred to a personal computer for conversion to .wav format and stored for further processing using the MATLAB software environment. The investigators “found that the amplitude of the smartphone-acquired sounds was highly correlated with the airflow from a spirometer” and that the increase in sound amplitude with flow followed a power law relationship. Additionally, and perhaps more importantly, the authors found that accurate respiratory rates can be obtained from tracheal sounds when the Shannon entropy of the tracheal sounds is used as an indicator of the onset of a breath (Fig. 2).
v A USB OTG (On-The-Go) adapter allows tablets or smartphones to act as a host, allowing other USB devices, such as flash drives, mice or keyboards, to be attached to them.
6. WAV, MP3 AND OTHER AUDIO RECORDING FORMATS
Computer files containing audio signals exist in a rich variety of formats. The specific digital layout of the audio data is called the audio coding format and comes in two types: uncompressed or compressed, the latter which can dramatically reduce the file size, but often at the price of some data loss. Uncompressed audio formats include the WAV, AIFF, and AU formats, of which the WAV audio format is by far the best known. A number of lossless compressed formats are also available, the FLAC format being amongst the best known. Amongst lossy compressed audio formats, the MP3 format is easily the best known and most commonly used for music applications. The Audacity audio editing package (Fig. 8) natively supports the WAV, MP3 and AIFF formats as well as numerous other formats through available extensions. That being said, most bio-acoustics researchers will be happy to use the WAV format for all their research work, as they are the standard audio file format used in Windows PCs and allow for CD-quality sound files. Note that the theoretical drawback of large WAV file sizes (around 10 MB per minute) is no longer a major issue given the enormous drop in data storage costs in recent years. For more information of audio file formats, the interested reader is directed to https://en.wikipedia.org/ wiki/Audio_file_format.
7. SOFTWARE FOR AUDIO SIGNAL PROCESSING
Investigators who are interested in processing breath sounds and other bioacoustical signals as part of their research have available to them a true cornucopia of options. In our work spanning two decades, we have used two inexpensive audio signal processing products, Goldwave (www.gold wave.com) and Spectrogram16 (no longer commercially available.) In recent years a large number of additional products have become available, although most are aimed at sound engineers and audio producers rather than bioacoustics researchers. For a list of free software for audio applications, visit https://en.wikipedia.org/wiki/Comparison_of_free_soft ware_for_audio. However, if one were to pick one free audio editor to master, we would recommend Audacity (Fig. 8), an open-source digital audio editor and recording application package available for the Windows, macOS/OS X and Linux operating systems. Audacity can be downloaded at https:// www.audacityteam.org/
8. ELECTRONIC PROCESSING OF BREATH SOUNDS
Numerous investigators have looked at ways of filtering breath sounds for particular clinical ends. For instance, a number have studied methods for the separation heart sounds from lung sound signals with the goal of getting better auscultation results [22-26]. Other investigators have applied digital techniques to characterize respiratory crackles [27-31] or to detect or characterize wheezes [32, 33].
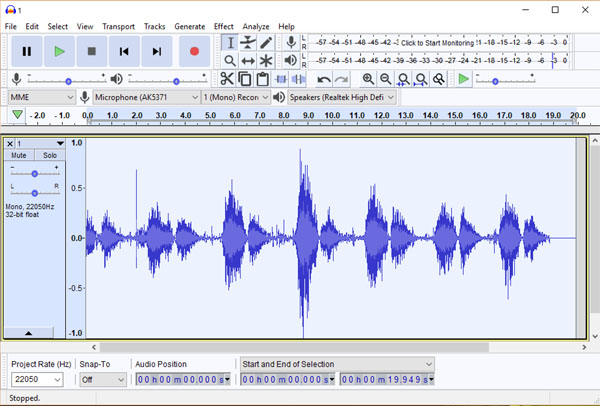
One of the most fundamental forms of electronic processing of breath sounds is frequency filtering. In the past, such filtering was done primarily using analog filters, but digital filtering methods are more commonly used now because their ease of use and minimal cost. In particular, the (free) Audacity audio signal processing system offers a number of digital filtering options that investigators may find to be attractive (vide infra).
9. THE MASIMO SYSTEM FOR ACOUSTICAL RESPIRATORY MONITORING
The Masimo Corporation (www.masimo.com) has successfully commercialized a form of respiratory acoustic monitoring that employs a flat adhesive acoustic sensor applied to the neck, near the trachea (Fig. 3) [34-40]. Known as Masimo Rainbow SET Acoustic Monitoring, the system reliably estimates the connected patient’s respiratory rate with the assistance of special proprietary software algorithms collectively known as Signal Extraction Technology® (SET®). While multiple studies have confirmed the value of this method of acoustical respiratory monitoring in a number of settings, the technology as currently available has some important limitations that should be explained. First, neither the raw nor the processed acoustic signal is available to the clinician to listen to, although the time-domain signal is displayed. The fact that the system does not provide an analog signal output for such purposes also limits the kind of supplementary analysis that might otherwise be performed, such as digitally recording the obtained signals, subjecting the signal to analog or digital filtering, or carrying out real-time color spectrographic analysis of the obtained breath sounds. Also, because of the proprietary nature of the Masimo acoustic monitoring system, little is publicly known about the flat acoustic sensor used. The likelihood, however, is that their sensor is based on piezoelectric film technology, as this technology has proven to be very useful in a variety of clinical applications [41-43].
CONCLUSION
Although a variety of methods are available for the assessment of patient breathing, bio-acoustical methods of respiratory assessment offer some special advantages. To this end we reviewed various clinical and technical matters pertaining to recording and analyzing breath sounds in the time-domain. Audacity, an open-source digital audio editor and recording package can be freely downloaded at https://www.audacityteam.org and is recommended for investigators seeking to conduct research on breath sound analysis.
LIST OF ABBREVIATIONS
| ECG | = Electrocardiogram |
| OTG | = On The Go |
| PACU | = Postanesthesia Care Unit |
| RR | = Respiratory Rate |
| SET | = Signal Extraction Technology |
| SPL | = Sound Pressure Level |
| USB | = Universal Serial Bus |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


