All published articles of this journal are available on ScienceDirect.
Epidural Clonidine as an Adjuvant to Local Anesthetic in Lower Abdominal and Lower Limb Surgeries: A Randomised Controlled Study
Abstract
Background:
Acute pain leads to adverse physiological and psychological disturbances. Hence, this study was done to evaluate and compare the onset and duration of sensory anesthesia, motor paralysis and duration of analgesia using 0.5% plain bupivacaine, with clonidine (2μg/kg) in patients posted for lower abdominal and lower limb surgeries under epidural anaesthesia.
Materials and methods:
62 Patients posted for elective lower abdominal, gynaecological and lower limb surgeries under epidural anesthesia, aged 18 to 60 years, height more than 150 cms of ASA physical status 1 or 2 were included. All patients were randomized into two groups of 31 individuals each.
Results:
Clonidine in the dose of 2μg/kg added to bupivacaine injected into epidural space significantly prolonged the duration of analgesia when compared to bupivacaine alone. No effect on the onset of sensory and motor blockade was observed. However, it increases the duration of motor blockade. Clonidine also has effect on sedation level, pulse rate and mean arterial blood pressure.
Conclusion:
Clonidine causes increased sedation; fall in pulse rate and mean arterial blood pressure, which however, did not require active intervention in this study.
1. INTRODUCTION
Attenuation of postoperative pain, especially with certain types of analgesic regimens, may decrease perioperative morbidity and mortality [1]. Acute pain results in potentially life-threatening problems [2]. Epidural anesthesia with local anesthetics, reduces physiologic responses to surgery and also provides superior pain relief [3]. Various adjuvants like opioids, epinephrine, clonidine, ketamine, neostigmine, adenosine, midazolam, magnesium, verapamil, ketorolac, etc. have been tried with local anesthetics in the epidural space, to enhance analgesia while minimizing side effects [4]. Clonidine being a partial α2 adrenergic agonist has antihypertensive effects and can potentiate effects of local anesthetics. It acts by opioids-independent mechanism, stimulates α2 adrenoreceptors reducing central neural transmission in spinal neurons, and inhibits the release of substance-P. It acts presynaptically interfering with nitric oxide mechanisms and protein kinases as well as by stimulation of cholinergic interneuron [5]. Anaesthesia was prolonged when clonidine was added to local anaesthetics for peripheral nerve blocks [6-12]. The analgesic effect of clonidine is more potent after neuraxial administration which points to a spinal site of action, thus favouring neuraxial (intrathecal or epidural) administration [13-16]. Epidural or intrathecal administration of clonidine potentiates the anesthetic action and reduces the dose requirement of volatile or injectable general or regional anesthetic agents with correspondingly fewer side effects [17-20].
2. MATERIALS AND METHODS
62 patients undergoing elective lower abdominal, gynaecological and lower limb surgeries under epidural anesthesia at Kasturba Medical College Hospital, Mangaluru were enrolled in the study. Ethics committee approval and written informed consent from patients aged between 18 to 60 years, height more than 150 cm of ASA physical status 1 or 2 were obtained. Patients with absolute or relative contraindications for epidural, ASA grade 3 or 4, with an adverse reaction to local anesthetics, on alpha-adrenergic receptor blockers, calcium channel blockers, ACE inhibitors, with body weight > 120 kg and height < 150 cm were excluded. Patients were randomized and allocated into 2 groups of 31 individuals each by sealed envelope method.
Group І: 0.5% bupivacaine + 0.9% normal saline, 1 ml.
Group ІІ: 0.5% bupivacaine + Inj. Clonidine 2μg/kg (1 ml).
The volume of bupivacaine injected depended on the type of surgery and level of block required (1.5 ml/segment).
A pre-anesthetic evaluation was done for all patients on the day prior to surgery regarding history, general physical examination and relevant investigations. Baseline vital signs were recorded. Pre-operative preparation included a period of overnight fasting. Patients were introduced to the Visual Analogue Scale (VAS) and were taught how to use it. Zero end of the scale was taken as no pain and 10 as maximum possible pain imaginable. Patients asking for pharmacological premedication were excluded from the study. This was done to assess the effect of study drug i.e., clonidine, on sedation levels. After patients were transferred to the operating room, an Intravenous (IV) access was secured in the non-dominant upper limb using an 18G IV cannula. 10-15 ml/kg of IV crystalloid solution was given over 15 minutes just before administering epidural anesthesia. Minimum mandatory monitors like a pulse oximeter, non-invasive blood pressure and electrocardiogram were used and baseline oxygen saturation (SpO2), Blood Pressure (BP) and pulse were noted. The patients were positioned in the lateral decubitus/ sitting position. Under absolute asepsis, L2-L3 interspace was punctured with an 18G Tuohy needle after infiltration of the skin and interspinous space with 2% plain lignocaine. Epidural space was identified with loss of resistance technique with saline. An 18G epidural catheter was then passed through the Tuohy needle and was left 4-5 cm into the epidural space in cephalad direction. A test dose of 3cc of 2% lignocaine with adrenaline 1:200,000 was given. BP, Pulse Rate (PR), Electrocardiograph (ECG) and SpO2 were monitored intraoperatively. PR, BP and SpO2 were measured at an interval of every 5 min up to 30 min, then at 15 min interval upto 60 min and thereafter at 30 min interval till the end of surgery. After confirming that there was no catheter misplacement, the control group(1) received 0.5% bupivacaine and 1 ml of 0.9% normal saline whereas the clonidine group (2) received 0.5% bupivacaine and 1ml of 2ug/kg of clonidine. During surgery, crystalloid intravenous fluids were administered at a rate of 150 ml/hr. Depending on perioperative blood loss and hemodynamic instability, additional IV fluids (crystalloids, colloids and blood) were administered.
2.1. Assessment of Sensory Block
Bilateral cephalad extension of anesthesia was assessed at each dermatomal level using temperature discrimination. Time of onset of sensory block was taken as the time from completion of epidural injection to the loss of temperature discrimination at the level desired for that particular surgery.
2.2. Analgesic Duration
VAS was used to assess pain as shown in the chart [21]. The intensity of pain was charted on VAS every hour and the duration to reach a VAS score of 6 (moderate pain) was noted for patients in both groups. Analgesic duration for a particular group was taken as the time for VAS score to reach 6 (moderate pain) from time of injection of epidural drug or for patients request for further analgesia from time of injection of the drug. Further analgesia was maintained through regular epidural top-ups or infusion of bupivacaine as deemed appropriate by the treating consultant.
2.3. Assessment of Motor Block
Modified Bromage scale (by Breen et al.) [22]. was used to assess motor block every minute till peak effect and then every 5 min after that. The time of onset of complete motor block was assessed as the time from completion of epidural injection to the establishment of grade 1 motor blockade. Duration of motor blockade was taken as time from the regression of bromage reading from 1 to 2 (complete block to almost complete block).
| Modified Bromage Scale | |
|---|---|
| Grade | Criteria |
| 1 | Complete block (unable to move feet or knees) |
| 2 | Almost complete block (able to move feet only) |
| 3 | Partial block (just able to move knees) |
| 4 | Detectable weakness of hip flexion while supine |
| 5 | No detectable weakness of hip flexion while supine with full flexion of knees |
2.4. Hemodynamics and Side Effects
Decrease in mean arterial blood pressure greater than 20% below preanesthetic baseline or systolic BP less than 90 mm Hg was treated with IV fluids and Inj. mephentermine 6-12mg IV. A decrease in HR was treated in accordance with the bradycardia algorithm of the American Heart Association. Side effects like nausea, vomiting, shivering or difficulty in micturition occurred. Inj. ondansetron 0.1 mg/kg IV was used to treat vomiting. Inj. butorphanol 0.5-1.0 mg IV. given to treat shivering. Degree of sedation was assessed using the Ramsay Sedation score [23]. In case butorphanol was administered to any patient for shivering, sedation score prior to administration of butorphanol was used for statistical purposes. No narcotics, analgesics or other CNS depressant drugs were given in addition to an epidural. On completion of the surgery, patients transferred to the postoperative ward and staff nurses were instructed to notify the anesthesiologist on call in case of:
(1) Respiratory rate lower than 8 breaths/ min.
(2) Oxygen saturation lower than 90%.
(3) Systolic blood pressure less than 90 mmHg.
(4) Inadequate analgesia (Patients with VAS more than 6 i.e severe to worst possible pain).
3. STATISTICAL ANALYSIS
SPSS 11.5 software was used to perform statistical analysis. The student’s unpaired T-test was used for the analysis of mean age, height and weight distribution, time of onset of sensory and motor block, duration of motor block and analgesia. A chi-square test was used for the analysis of intraoperative side effects. P-value < 0.05 was considered statistically significant.
4. RESULTS
The age, weight and height were comparable in both the groups with no significant statistical differences (Table 1).
In this study, MAP in mm Hg preoperatively was 98.06 ± 7.12 and 97.77 ± 5.33 in control and clonidine group, respectively, which was not statistically significant.
Changes in Mean Arterial blood Pressure (MAP) at 60 mins after administration of the epidural drug was 83 ± 10.63 and 75.03 ± 9.06 in control and clonidine group, respectively. This fall in mean arterial blood pressure was statistically significant in the clonidine group (p-value= 0.002) (Table 2).
A decrease in pulse rate was statistically significant (p-value = 0.026) after 30 mins of drug administration in the clonidine group (71.94 ± 11.89) as compared to the control group (74.03 ± 9.74) (Table 3).
No significant difference in the onset of sensory and motor block in either of the groups. The clonidine group had a significant prolongation of the motor block (p-value < 0.001). Duration of motor blockade (in mins) was 108 ± 11.15 and 229 ± 44.27 in the control and clonidine group respectively (Table 4).
The analgesic duration was significantly prolonged in the clonidine group (p-value < 0.001) Duration of analgesia (in mins) was 136 ± 11.74 and 380 ± 63.01 in control & clonidine groups, respectively (Table 5).
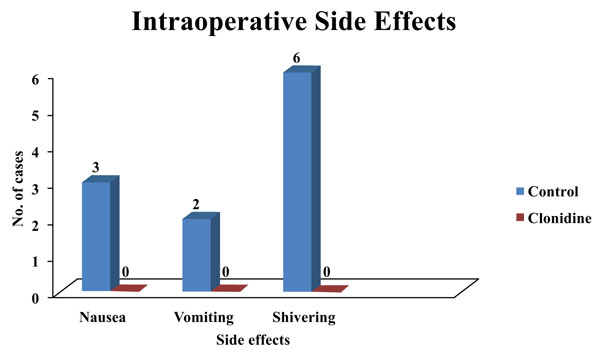
Three patients had nausea and two patients had vomited in the control group. However, no significant difference was observed in the incidence of nausea and vomiting in either group. The control group had 19.4% of patients exhibiting shivering which was statistically significant (Fig. 1).
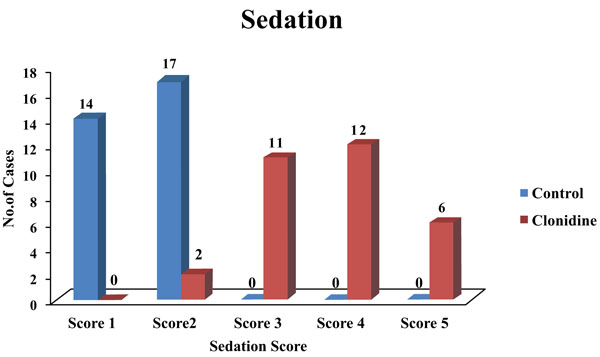
The clonidine group had patients with a sedation score of 3 and above, which was statistically significant (Fig. 2).
5. DISCUSSION
Synergistic antinociceptive effect with opioids is known to occur with clonidine, an α2 adrenergic agonist. Clonidine, an α2 agonist, produces analgesia with a non- opioid mechanism [24]. Clonidine does not affect proprioception like local anesthetics. Respiratory depression, itching, nausea and vomiting, which occur with opioids, are not seen with clonidine [24]. The potency of clonidine as an analgesic increased when given epidurally. Side effects like hypotension, bradycardia and sedation can occur. α2 agonists affect the descending noradrenergic tract in the spinal cord that helps in pain modulation via a non-opioid mechanism. All the patients in this study were comparable with respect to demographic profiles. In our study, there was a significant fall in mean arterial pressure at an interval of 60 min (p-value = 0.002) after the epidural injection of the drug in the clonidine group (75.03 ± 9.06) as compared to control group (83.00 ± 10.63). Ikeda et al. in their study in 2003, concluded that epidural administration of clonidine causes hypotension and bradycardia due to sympathetic blockade [25]. Rockemann et al. in their research in 1995, concluded that hemodynamic alteration after epidural clonidine was caused mainly by a fall in heart rate [26]. In our study, decrease in pulse rate was statistically significant (p-value = 0.026) after 30 minutes of drug administration in the clonidine group (71.94 ± 11.89) as compared to the control group (74.03 ± 9.74).
Intrinsic antihypertensive action of clonidine was known to cause this and not the effect of analgesia. In this study, the duration of analgesia was very much prolonged in the clonidine group (380.84 ± 63.01) as compared to the control group (136.06 ± 11.74). Parker et al. in 2007 studied increased analgesic duration on the addition of clonidine to bupivacaine continuous epidural infusion [27]. Huang et al. 2007 conducted a study on patients undergoing total knee arthroplasty, who received clonidine for patient-controlled epidural analgesia [28]. Less postoperative pain was seen in clonidine groups. Giovanni Cucchiaro in 2006 compared the incidence of vomiting and pruritus as well as the analgesic profile and sedation score of three different combinations of bupivacaine, fentanyl, and clonidine administered epidurally in patients undergoing Nuss procedure [29]. He found no significant difference in the sedation score. He also found that the number of patients who experienced vomiting was significantly less in the clonidine group. The number of patients who experienced pruritus was significantly less in the clonidine group versus the other groups. In our study, the clonidine group had a sedation score of 3 and above as compared to patients in the control group which was statistically significant.
No patients in the clonidine group experienced nausea and vomiting, whereas 9.7% of patients experienced nausea, 6.5% of patients experienced vomiting in the control group in our study.
| Parameters | Group 1 (Control) | Group 2 (Clonidine) |
| Age (in years) | 49.94 (±8.25) | 47.10 (±9.27) |
| Weight (in Kg) | 54.48 (±9.82) | 54.58 (±8.67) |
| Height (in cm) | 165.26 (±4.32) | 164.32 (±4.42) |
| Sex (M:F) | 13:18 | 11:18 |
| Time | Control | Clonidine | p-value | Inference |
| Pre-op | 98.06 ± 7.12 | 97.77 ± 5.33 | 0.86 | N.S. |
| 5 min | 95.00 ± 6.54 | 93.10 ± 6.13 | 0.24 | N.S. |
| 10 min | 87.87 ± 9.01 | 85.06 ± 7.56 | 0.19 | N.S. |
| 15 min | 83.26 ± 7.99 | 83.48 ± 6.05 | 0.90 | N.S. |
| 20 min | 81.00 ± 7.80 | 79.26 ± 5.91 | 0.33 | N.S. |
| 25 min | 79.45 ± 8.72 | 77.71 ± 7.06 | 0.39 | N.S. |
| 30 min | 78.68 ± 8.35 | 76.71 ± 9.25 | 0.38 | N.S |
| 45 min | 80.29 ± 9.40 | 76.65 ± 9.23 | 0.13 | N.S |
| 60 min | 83.00 ± 10.63 | 75.03 ± 9.06 | 0.002 | S |
| 90 min | 85.45 ± 9.50 | 76.94 ± 6.99 | 0.001 | S |
| 120 min | 87.71 ± 8.90 | 77.32 ± 8.10 | 0.001 | S |
| 150 min | 89.06 ± 15.09 | 77.45 ± 6.17 | 0.001 | S |
| Time | Control | Clonidine | P value | Inference |
| Pre-op | 82.65 ± 10.94 | 84.29 ± 12.90 | 0.056 | N.S. |
| 5 min | 81.45 ± 12.68 | 80.10 ± 12.01 | 0.053 | N.S. |
| 10 min | 80.41 ± 10.43 | 79.58 ± 10.91 | 0.057 | N.S. |
| 15 min | 78.12 ± 10.30 | 78.32 ± 12.38 | 0.053 | N.S. |
| 20 min | 76.71 ± 9.72 | 77.19 ± 12.72 | 0.21 | N.S. |
| 25 min | 74.94 ± 9.35 | 74.45 ± 12.54 | 0.11 | N.S. |
| 30 min | 74.03 ± 9.74 | 71.94 ± 11.89 | 0.026 | S |
| 45 min | 71.39 ± 9.97 | 67.48 ± 11.84 | 0.031 | S |
| 60 min | 70.94 ± 11.02 | 65.77 ± 9.81 | 0.023 | S |
| 90 min | 70.32 ± 11.10 | 64.46 ± 9.18 | 0.01 | S |
| 120 min | 70.42 ± 10.60 | 62.16 ± 9.40 | 0.043 | S |
| 150 min | 71.97 ± 10.70 | 61.39 ± 9.67 | 0.035 | S |
| Time in Minutes | No. of Patients | |
| Control | Clonidine | |
| 50-100 | 12 | 00 |
| 101-150 | 19 | 00 |
| 151-200 | 00 | 08 |
| 201-250 | 00 | 14 |
| 251-300 | 00 | 06 |
| 301-350 | 00 | 03 |
| Minimum time | 90 | 180 |
| Maximum time | 130 | 315 |
| Mean ± S.D. | 108 ± 11.15 | 229 ± 44.27 |
| Time in Minutes | No. of Patients | |
| Control | Clonidine | |
| 101-150 | 30 | 00 |
| 151-200 | 01 | 00 |
| 201-250 | 00 | 01 |
| 251-300 | 00 | 00 |
| 301-350 | 00 | 11 |
| 351-400 | 00 | 09 |
| 401-450 | 00 | 06 |
| 451-500 | 00 | 04 |
| Minimum time | 120 | 250 |
| Maximum time | 165 | 465 |
| Mean ± S.D. | 136 ± 11.74 | 380 ± 63.01 |
CONCLUSION
There was an increase in the duration of analgesia following the addition of clonidine to bupivacaine 2 ug/kg in comparison to bupivacaine alone when instilled in the epidural space. The duration of motor block was increased with no effect on the onset of sensory and motor block.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethics approval was obtained by Institutional Ethics Committee Kasturba Medical College Hospital, Mangaluru, India.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975. as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was taken from all patients.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


