All published articles of this journal are available on ScienceDirect.
Evaluation of Efficacy of Free Opioid Anesthesia for Laparoscopic Cholecystectomy: A Prospective, Randomized Double-blinded Study
Abstract
Objectives:
To evaluate efficacy and side effects of free opioid anesthesia for laparoscopic cholecystectomy.
Methods:
A prospective study was performed on 94 patients undergoing laparoscopic cholecystectomy in Military Hospital 103 from May 2018 to February 2019. These patients were randomly allocated into two groups: patients in FOA (free - opioid anesthesia) group were administered lidocaine (2 mg/kg before induction and 1.5 mg/kg/h for maintenance), magnesium (30 mg/kg before induction and 1.5 g infusion for maintenance) combined with Intravenous (IV) injection of ketamine (0.5 mg/kg), and ketorolac (30 mg); while patients in OA group (opioid anesthesia) were provided with IV fentanyl (5 mcg/kg for induction and 1.5 mcg/kg every 30 minutes for maintenance of anesthesia). Both groups received total intravenous anesthesia by propofol. The depth of anesthesia was monitored by the entropy module during surgery. Neuromuscular blockade was reversed by sugammadex 2 mg/kg at the end of surgery. The postoperative analgesia was delivered using IV fentanyl for 48 to 72 hours. Visual Analog Scale (VAS) score was measured 10 mins, 20 mins, 1 hour, 2 hours and 3 hours after surgery.
Results:
All patients had an excellent quality of anesthesia with RE (Respond Entropy), SE (State Entropy) always under 60 from induction to abdominal closure without intraoperative awareness and postoperative recall of the operation; 100% of the patients were extubated immediately after surgery. In the first three postoperative hours fentanyl consumption in Group FOA was significantly lower than in Group OA (31.91 ± 3.98 mcg versus 34.47 ± 7.17 mcg, p=0,035). In the OA group, the rate of intraoperative hypotension was higher compared to its counterpart. Despite the higher risk of hypersalivation, group FOA had a significantly lower incidence of nausea and vomiting.
Conclusion:
Free opioid anesthesia provided adequate sedation and amnesia and may be an alternative approach to opioid anesthesia for laparoscopic cholecystectomy. Patients under free opioid anesthesia experienced a lower incidence of intraoperative hypotension, lower rate of nausea, vomiting and lower demand for analgesia in the early postoperative period (0 - 3 h) compared to those receiving opioid anesthesia.
1. INTRODUCTION
Opioids have been used as one of three basic components ofbalanced anesthesia, including anesthetic drugs, pain relievers, and neuromuscular blockade agents. Opioids not only facilitate deep anesthesia but also create the most favorable conditions for surgeries, especially laparoscopic surgery. Fentanyl is a potent opioid used to control pain, reduce the dose of sympathomimetic inhibitors and maintain hemodynamic stability [1]. However, several common side effects of fentanyl are well known: nausea and vomiting, constipation, urinary retention, headache, pruritus, rash, histamine release, biliary spasm and respiratory depression, the most severe adverse effect. In one study, the percentage of postoperative opioid-related side effects in patients using opioids was reported at 2.67% and there was an association between opioids consumption and the increase in the length of hospital stay as well as health care costs [2]. Patients taking high doses of fentanyl during surgery always require higher doses of opioids in the postoperative period than those using lower doses [3].
Free Opioid Anesthesia (FOA) is being used in many countries around the world, making the use of multimodal pain therapy, which includes local anesthesia combined with NSAIDs and sympathetic block in surgery. This method was demonstrated to contribute to Enhanced Recovery After Surgery (ERAS) [1]. The use of long-acting local anesthetics also enhances postoperative pain relief. As a result, the concept of balanced anesthesia now has a change in three basic components: hypnotics, muscle relaxants and sympathomimetic inhibitors [4]. The research purposes were to evaluate the anesthetic quality, effects on circulation, respiration and side effects of non-opioid anesthesia for laparoscopic cholecystectomy.
2. MATERIALS AND METHODS
A prospective, randomized, double-blinded study was performed on 94 patients undertaking laparoscopic cholecystectomy at Military Hospital 103 from May 2018 to February 2019. Excluded criteria took account of disagreement to enroll in the study; a history of epilepsy, mental illness, communication difficulties; a history of increased intracranial pressure; heart disease, hypertension; bradycardia; liver failure, kidney failure; pregnancy or nursing mother. There were no patients excluded from the study.
We used 94 opaque sealed envelopes to perform randomization, 47 for each group. Before entering the operating room, the patient randomly picked up one in 94 envelopes which indicated group assignment and described the study protocol. An anaesthesiologist opened the envelope and prepared medication depicted in the envelope. The anaesthesiologist did not take part in perioperative anesthetic management as well as data collection and analysis.
Preoperative preparations consisted of completing standardized laboratory tests (blood count, coagulation test, hepatic and renal function, respiratory and circulatory status…). Nihon Kohden monitoring system such as blood pressure, SpO2, electrocardiogram; TOF (train-of-four) scan monitor and Entropy was applied.
Intubation was implemented when TOF=0 and RE, SE ≤ 60, then mechanical ventilation was set at a tidal volume 8-10 ml/kg with the volume-controlled mode and respiratory rate at 12-14 breath/minute. Peak airway pressure was maintained within the range of 12-16 cmH2O and EtCO2 (End-tidal Carbon dioxide) was kept from 25 to 35 mmHg with fresh gas flow of 1.2 - 2 liters/minute. When surgeons performed CO2 insufflation, ventilation variables were modified to keep EtCO2 <40 mmHg and airway pressure <30 cm H2O.
As far as the anaesthesia protocol was concerned, 94 patients were randomized into 2 groups:
47 patients In FOA Group (Free - opioid anesthesia) were injected intravenously lidocaine 2 mg/kg and magnesium 30 mg/kg before induction. Then intravenous propofol 2 - 2.5 mg/kg; ketogesic 30 mg and rocuronium 0.8 mg/kg were utilized for induction. Right after induction, patients were given a bolus intravenous dose of ketamine 0.5 mg/kg coupled with local anesthesia at the edge of the incision by ropivacaine 0.5%. In the maintenance phase, propofol was infused continuously with dose adjusted to maintain RE, SE within 40 - 60. Simultaneously, intravenous infusion of lidocaine at the rate of 1.5 mg/kg/h and intravenous infusion of magnesium 1.5 g were applied. When the gallbladder was resected, intravenous infusion of 1g paracetamol was supplemented. 10 mg of rocuronium was repeated when TOF ≥ 2 twitch (no last injection if the estimated duration from the point of injection to the point of abdominal closure was less than 20 minutes).
47 patients In OA Group (Opioid anesthesia) underwent induction with IV propofol 2-2.5 mg/kg; fentanyl 5mcg/kg and rocuronium 0.8 mg/kg. Then, maintenance was performed employing continuous infusion of propofol with dose adjusted to maintain RE, SE within 40-60. 10 mg of rocuronium was repeatedly administered when TOF ≥ 2 twitch (no last injection when the estimated duration from the point of injection to the point of abdominal closure is shorter than 20 minutes). 1.5 mcg/kg of fentanyl was repeated every 30 minutes.
The drugs were administered in 10 mL and 50 mL syringes labelled as “loading” or “infusion” respectively. All the drugs delivered in loading dose (lidocain, magnesium, ketogesic and ketamine in FOA group; fentanyl and normal saline in OA group) were diluted in normal saline to 10 ml volume labeled undistinguishable as “loading-1” and “loading-2”, “loading-3”, “loading-4”. Because the number of loading drugs in the FOA group was greater than in OA group, we added some 10 ml normal saline syringes in the OA group to make the number of “loading” syringes in the two groups equal, thereby ensuring complete blinding. The infusion drugs (lidocaine and magnesium in FOA group or normal saline in OA group) were prepared in 50 mL syringes and labeled as “infusion-1” and “infusion-2”, respectively.
In both groups, neuromuscular blockade was reversed with sugammadex 2 mg/kg when stimulus T2 in TOF emerged. Postoperative analgesia was applied using continuous infusion of fentanyl 10 mcg per hour and bolus dose of 10 mcg fentanyl whenever VAS (Visual Analog Scale) scores at rest ≥4.
We compared the two groups on the basis of several variables including general characteristics such as age, gender, BMI (Body Mass Index), surgical duration, duration of anaesthesia, and duration of induction; total drug dosages of propofol (mg), lidocaine (mg), magnesium (mg), ketamine (mg), rocuronium (mg), sugammadex (mg) and especially dose of fentanyl; impact on the circulatory and respiratory system (heart rate, blood pressure, SpO2, EtCO2); the sedation effect (Entropy, intraoperative awareness and ability of postoperative recall of procedure) at 9 time points: in operating room, pre-induction, after induction, pre- incision, after incision, before closure, after closure, before extubation, after extubation (abbreviated to T1, T2, T3, T4, T5, T6, T7, T8, and T9); side effects: vomiting, nausea, shivering, allergies, hypersalivation, hypertension, hypotension. Visual Analog Scale (VAS) score was measured 10 mins, 20 mins, 1 hour, 2 hours and 3 hours after surgery.
The data was collected, analyzed and processed by medical statistics using SPSS 20.0 software. Quantitative variables were described as the means ± standard deviations (± SD). Discrete variables were described in percentages. The results are presented in tables and graphs.
3. RESULTS
3.1. Patient Characteristics
Table 1 shows the demographics of the 94 patients completing the study. There were no differences in gender, age, BMI and ASA classification of physical status.
3.2. General Anesthesia and Surgical Characteristics
Table 2 illustrates the characteristics of the anesthesia process and surgery. There were no differences between the two groups in duration of induction, duration of anaesthesia, surgical duration, time to achieve TOF=0.5; TOF=0.7 and TOF=0.9 and extubation time, the consumption of propofol, rocuronium, and sugammadex.
3.3. Sedation Effect
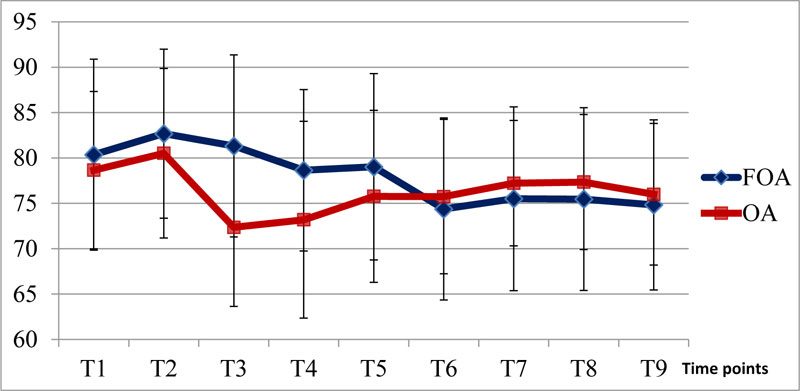
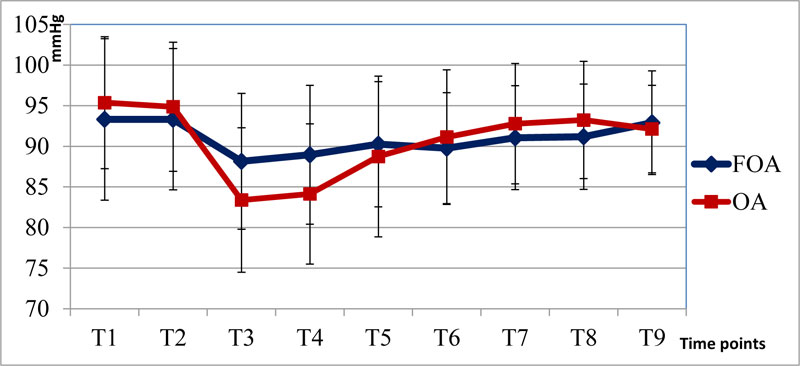
The level of sedation measured by Entropy (SE and RE) did not differ among the two groups during the perioperative period. The heart rate and mean blood pressure were only significantly different at T3 and T4 (Figs. 1 and 2).
| Group/ Characteristics |
Group FOA (n=47) |
Group OA (n=47) |
p |
|---|---|---|---|
| Gender: male/female | 19/28 | 19/28 | 1.000 |
| Age (years) | 49.81 ± 16.01 [16 - 82] | 54.81 ±15.22 [21 - 77] | 0.124 |
| BMI (kg/m2) | 20.4 ± 2.4 [15.78 - 25.39] | 21.71 ± 2.50 [17.67 - 28.89] | 0.977 |
| ASA: I/II/III | 37/8/2 | 40/5/2 | 0.667 |
| Group/ Characteristics |
Group FOA (n=47) |
Group OA (n=47) |
p | |
|---|---|---|---|---|
| Duration of induction (minutes) | 8.28 ± 0.52 [7 - 9.75] |
8.24 ± 0.54 [7.18 - 9.52] |
0.717 | |
| Duration of general anesthesia (minutes) | 80.53 ± 25.65 [35 - 130] |
78.51 ± 23.31 [45 - 125] |
0.69 | |
| Duration of surgery (minutes) | 74.30 ± 24.46 [32 - 120] |
73.98 ± 23.83 [35 - 120] |
0.949 | |
| TOF index (minutes) | ||||
| TOF=0,5 TOF=0,7 • • • TOF=0,9 |
1.73 ± 0.54 [0.5 - 2.55] 2.50 ± 0.67 [0.87 -3.80] 3.07 ± 0.72 [1.08 - 4.18] |
1.70 ±0.46 [0.7 - 2.62] 2.51 ± 0.52 [1.43 - 3.47] 3.10 ± 0.61 [1.68 - 4.03] |
0.715 0.977 0.860 |
|
| Extubation time (minutes) | 3.22 ± 0.72 [1.13 - 4.30] | 3.25 ± 0.62 [1.98 - 4.18] | 0.819 | |
| Dose of drugs | Propofol (mg) | 509.34 ± 165.74 [310 - 975] |
510.19 ± 149.18 [280 - 866] |
0.979 |
| Rocuronium (mg) | 52.66 ± 10.15 [35 - 80] |
52.77 ± 10.97 [35 - 80] |
0.961 | |
| Suggamadex (mg) | 108.72 ± 14.98 [80 - 140] |
110.21 ± 16.35 [80 - 150] |
0.646 | |
3.4. Side Effects
The side effects are presented in Table 3. Despite the higher risk of hypersalivation, Group FOA had a significantly lower incidence of nausea and vomiting compared to group OA.
3.5. Postoperative Analgesia
There were no differences in VAS score between all time points during the early postoperative period (0 - 3h).
Postoperative fentanyl consumption: the postoperative fentanyl consumption was shown in Table 4. Dosages of extra fentanyl and total fentanyl, as well as the numbers of fentanyl extra injection used during 3 hours after surgery in group OA were significantly higher than group FOA.
4. DISCUSSION
Enhancing recovery after surgery has gained increasing concern of anesthesiologists and surgeons to improve the quality of care for patients. The use of opioid replacement drugs and NSAIDs contributes to reducing treatment costs and promoting patients' recovery [5]. The clinical practice shows that patients with general anesthesia and high doses of fentanyl - a potent opioid always require higher doses of opioids in the postoperative period that cause Opioid-Induced Hyperalgesia (OIH) [3]. Therefore, Free-opioid Anesthesia (FOA) has been introduced in many countries across the world.
Our study compared the free - opioid anesthesia using lidocaine, magnesium, propofol infusion combined with intravenous low doses of ketamine, ketorolac, postoperative infusion of acetaminophen and local anesthesia at the edge of the incision with ropivacaine 0.5% to opioid-based anesthesia performed by using fentanyl and propofol infusion. The results indicated that the heart rate and mean blood pressure at T3 and T4 in group OA were significantly lower than in group FOA, with p<0.05.
|
Group Side Effects |
Group FOA (n=47) |
Group OA (n=47) |
p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Nausea | 3 | 6.38 | 9 | 19.15 | 0.005* |
| Vomiting | 0 | 0 | 5 | 10.64 | |
| Hypersalivation | 9 | 19.15 | 2 | 4.26 | |
| Hypotension | 1 | 2.13 | 8 | 17.02 | |
|
Group Dosages |
Group FOA (n=47) |
Group OA (n=47) |
P |
|---|---|---|---|
| Dosages of extra fentanyl (mcg) | 1.91 ± 3.98 [0 - 10] |
4.47 ± 7.17 [0 0 20] |
0.035* |
| Dosages of total fentanyl (mcg) | 31.91 ± 3.98 [30 - 40] |
34.47 ± 7.17 [30 0 50] |
0.035* |
| Numbers of injection (times) | 0.19 ± 0.40 [0 - 1] |
0.45 ± 0.72 [0 0 2] |
0.035* |
Intravenous lidocaine suppresses spontaneous impulses originating from injured nerve fibers and proximal dorsal root ganglions and functions by inhibition of NA-channels, NMDA (N-methyl-D-aspartate) and G-protein coupled receptors. It was useful to reduce opioid consumption, opioid- related side effects and pain intensity, as well as to decrease the incidence of postoperative ileus [6]. Magnesium acts as non-competitive antagonist of NMDA glutamate receptors, thus preventing depolarization and transmission of pain signals. Intraoperative use of magnesium can also reduce opioid consumption in the first postoperative 24 hours [7]. Ketamine is an NMDA antagonist of which low doses (<0.5 mg / kg) has the ability to diminish the requirement of postoperative analgesia by attenuating up to 25% of pain intensity within 48 hours after surgery [8].
Javier confirmed that free-opioid anesthesia was the better choice in some cases such as narcotic abuse history, morbidly obese patients with obstructive sleep apnea, opioid intolerance, and history of chronic diseases (immunodeficiency, cancer) [8]. In another study performed by Mansour et al.. (2013) on 28 obese patients (BMI>50 kg/m2) undergoing laparoscopic sleeve gastrectomy, the patients received either opioid or non-opioid-based general anesthesia. The results showed that non-opioid approach was as effective as the opioid counterpart but the non-opioid anesthesia was better at relieving pain in the postoperative period. However, Mansour only used relatively high doses of ketamine (0.5 mg/kg for induction and 0.5 mg/kg/h for maintenance), so the hallucinations appeared more frequently in this group compared to the opioid group [9]. The other studies of Feld (2003) and Yalcin Cok (2017) on obese patients also revealed similar results, confirming the effectiveness of free - opioid anesthesia. The difference among these studies lies in the combination of sympathomimetic stabilizers, intravenous anesthetic agents, the use of ketorolac, the dose of ketamine and thus level of hallucinations [10, 11].
In our study, both groups experienced safe and adequate general anesthesia with RE and SE always under 60 and there was no case of intraoperative awareness and postoperative recall of the operation in both groups. A Vakkuri et al. (2004) confirmed that Entropy ranging from 40 to 60 denote an adequate level of anesthesia without intraoperative awareness, like the Bispectral index [12]. After surgery, the patients were used sugammadex 2 mg/kg for muscle relaxation reversal and extubated when TOF ≥ 0.9 and other standards for endotracheal extubation was met. The results showed that recovery time to reach TOF = 0.5; 0.7; 0.9 and the extubated duration in patients used sugammadex were similar to the result of other studies in which the blockade was also reversed by sugammadex after total anesthesia [13]. Side effects in our study were significantly different between the two groups. The free-opioid group had a lower incidence of nausea and vomiting compared to the opioid group but a higher rate of hypersalivation, which could be eliminated by suction without serious complications in the postoperative period.
According to Mulier et al.. (2017), combining multimodal sympathetic nervous system stabilizers including α2 - adrenoreceptor agonists (clonidine, dexmidetomidine), intravenous local anesthetics (lidocaine, procaine), and magnesium can completely replace opioid used in general anesthesia which causes long-term sedation and OIH after surgery [14]. The author also confirmed the capability of significantly reducing opioid-related side effects, especially postoperative nausea and vomiting and respiratory depression [5, 14]. In another study on 5061 patients experiencing gastroscopy, Mulier found that free-opioid anesthesia with the combination of clonidine, dexmedetomidine, lidocaine, magnesium and less than 50 mg ketamine applied for 2337 patients resulted in the same efficacy but significantly decreased rate of complications as well as reduced postoperative analgesic morphine doses in comparison with 264 patients who received Low-Opioid Anesthesia (LOA) and 2451 patients applied opioid anesthesia [15]. The study of Mefkur Bakan et al. (2015) on 80 patients undergoing laparoscopic cholecystectomy showed that the rate of side effects such as vomiting, nausea, hypotension, and tremor in the opioid - remifentanil (RF) group was higher than in the free-opioid anesthesia group (DL), particularly the postoperative ephedrine, ondansetron, and fentanyl consumption during the first 2 hours after surgery in the RF group was significantly higher than in the DL group [16].
Another disadvantage of receiving prolonged or high daily opioid doses is acute opioid tolerance, a phenomenon in which exposure to a drug results in the diminishing of an effect or the need for a higher dose to maintain an effect. Tolerance can be divided into two main classifications: innate or acquired. Innate tolerance is related to pharmacogenetic makeup. Whereas, acquired tolerance is a consequence of repeated drug exposure, and can be classified into three general types according to the primary mechanisms: pharmacokinetic, pharmacodynamic, or learned. Pharmacodynamic tolerance occurs when the intrinsic responsivity of the receptor system diminishes over time. Acute tolerance is mediated predominantly by pharmacodynamic mechanisms, manifested as a decreased response following a single administration of the agent or during repeat-dosing but over a short time frame. This can usually be overcome with higher doses of opioids, although the risk of opioid-related adverse effects increases with this strategy. In our study, despite similar satisfaction levels between two groups with the same VAS score at rest and cough during the first three hours after surgery, there was larger postoperative fentanyl consumption in group OA (34.47 ± 7.17 mcg) compared to group FOA (31.91 ± 3.98 mcg) with p < 0.05. It might be due to the use of fentanyl in general anesthesia which causes opioid-induced hyperalgesia and/or tolerance in the postoperative period. Similarly, Chia et al.. comparing intraoperative use of high and low dose of fentanyl on the basis of pain scores and postoperative fentanyl consumption after hysterectomy draw a conclusion that the figure for the previous group was significantly higher [3]. In research of Mefkur Bakan et al. (2015), the postoperative fentanyl consumption during the first 2 hours after surgery was 120 ± 94 mcg in group RF, which was significantly higher than the figure for group DL with 75 ± 56 mcg. In addition, the number of patients who needed rescued analgesia was significantly higher in group RF compared to the statistics for group DL [16]. In another study on 5061 patients suffering from gastroscopy, J Mulier et al. (2016) revealed that FOA and LOA groups required less postoperative analgesic morphine doses on day 0 (6 mg and 15 mg) compared to group OA (26 mg), and the difference was significant with p<0.001 [15].
CONCLUSION
Free opioid anesthesia provided adequate sedation and amnesia with intraoperative RE and SE always being below 60 and no case suffering from intraoperative awareness and postoperative recall of the operation, so may be an alternative approach to opioid anesthesia for laparoscopic cholecystectomy. Patients under free opioid anesthesia experienced a lower incidence of intraoperative hypotension, lower rate of nausea, vomiting and lower demand for analgesia in the early postoperative period (0 - 3 h) compared to those receiving opioid anesthesia.
LIMITATIONS OF STUDY
There exist some limitations to our study. Firstly, according to the formula to calculate the sample size, the number of patients in each group has to be at least 54 patients. However, due to the restriction of time since this is a resident thesis, an adequate sample size could not be obtained. Secondly, Ketamine was shown by Maksimow, Sarkela (2006) to increases the high frequency EEG activity and thus make the Entropy monitor unpredictable. Therefore, the evaluation of RE and SE may need to be modulated. We did not consider this phenomenon in the study.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research was conducted following the guidelines and approval of VietNam Military Medical University Clinical Researches Ethics Committee under the approval number 2827/QĐ-HVQY.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all the participants of the work.
AVAILABILITY OF DATA AND MATERIALS
All data and materials are available in hospital's registry.
FUNDING
None.
CONFLICT OF INTEREST
All the authors certified that we had no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in the manuscript.
ACKNOWLEDGEMENTS
Declared none.


