All published articles of this journal are available on ScienceDirect.
Comparing Intravenous Single Low Doses of Magnesium Sulphate versus Dexamethasone as Adjuvants to Ultrasound Guided Transversus Abdominis Plane (TAP) Block for Prolongation of Postcesarean Analgesia
Abstract
Introduction:
The TAP block is a regional anesthetic technique, which blocks neural afferents between T6 and L1, which provide anterior abdominal walls and therefore help to alleviate postoperative pain.
Aim:
The aim is to compare the efficacy of preoperative single low dose of intravenous MgSO4 versus intravenous dexamethasone as adjuvants to ultrasound guided TAP block for prolongation of postcesaren analgesia.
Materials and Methods:
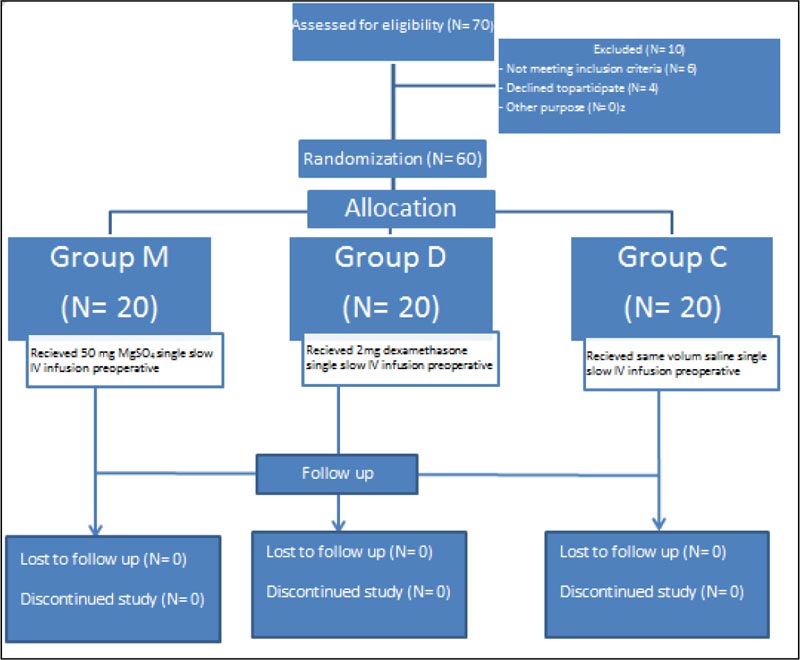
A total 60 pregnant females were selected undergoing elective caesarean sections under general anesthesia with ultrasound-guided transversus abdominis plane (TAP) block done at the end of surgery. Patients were randomly and equally allocated into three groups of 20 patients each. The first group of patients were classified as magnesium sulphate group (M)who received 50 mg/Kg IV, the second group of patients were classified as dexamethasone group (D) who received 2 mg IV and the third group was classified as the placebo group (C) who received IV saline.
Results:
Comparison of the VAS at 6 and 12 hours postoperatively showed statistically significantly lower values in group (M) and group (D) compared to group (C) and also group (M) was significantly lower than group (D) as well. The time interval until first rescue analgesia (Nalbuphine) needed by the patients (VAS ≥ 50) was significantly longer in group (M) compared to group (D) and group (C) consecutively. Additionally, it was significantly longer in group (D) than in the control group(C). The total dose of rescue analgesia consumed during the first 24 hours postoperatively was significantly lower in groups (M) than in group (D) and both groups showed lesser doses compared to group (C)
Conclusion:
We concluded that both MgSO4 and dexamethasone could prolong the postoperative duration and analgesic efficiency provided by the TAP block in cesarean sections. This further reduced the demands for postoperative rescue analgesia, with MgSO4 found to be more efficient than IV dexamethasone.
Clinical Trial Registration Number: NCT04223128
1. INTRODUCTION
Post-caesarean pain is usually classified as moderate to severe by most patients and inadequate treatment may affect mother-baby bonding, care of baby, and breastfeeding [1]. It may even increase the risk of thrombo-embolism as a result of inactivity due to pain [2]. The pain management should be adequate and safe for the breastfeeding baby. The pain of caesarean section essentially has two components somatic (due to abdominal wall incision), which represents the major part of experienced pain and visceral (from the uterus) [3].
Multimodal analgesic regimens include drugs such as opioids, local nerve blocks aiming to alleviate post-operative pain intensity and duration [4]. The Transversus Abdominis Plane (TAP) block is a regional anesthetic technique, where the thoracic sensory nerves from T6 to L1 provide sensory supply for the anterior abdominal wall [5]. Ultrasound guidance of TAP block provides a more efficient and safer approach than blind technique because it allows the local anesthetic to be more precisely located and deposited, with decreased risk of complications than conventional anatomical landmark techniques [6, 7].
Magnesium sulphate (MgSO4) is used as an adjuvant for regional anesthesia, offered in a number of routes, with one adjuvant being the standard, low-dose intravenous (50 mg/kg) [8]. The analgesic mechanism of MgSO4 is unknown, but it has been shown that interaction with calcium channels and N-methyl-D-aspartate (NMDA) receptors is significant. NMDA antagonism inhibits nociceptive central sensitisation and decreases the release of catecholamine with sympathetic activation and thus decreases peripheral nociception and stress responses to airway manipulations and surgery [8].
Dexamethasone has been proved to have significant postoperative analgesic benefits when administered as a single perioperative dose in two meta-analysis studies which were attributed to its potent anti-inflammatory effect [9, 10]. It is well known that IV dexamethasone at an intermediate dosage of 0.1-0.21 mg/kg has an opioid-sparing effect when applied to the multimodal analgesic protocol [11, 12]. Despite local anesthetics potentiation and prolongation of its analgesic duration exerted by dexamethasone when used as an adjuvant to Local Anesthetics (LA) in peripheral nerve block [13], it is not approved for the perineural route by the Food and Drug Administration, as many in vitro research works have proved that there is a high risk of peripheral neurotoxicity [14]. Intravenous dexamethasone has also been shown to be just as effective as the perineural route and reduce the need for post-operative analgesics in orofacial, urological and orthopedic operations [15]. Different doses of perineural or intravenous dexamethasone have been tried in different studies, but the optimal dose that can extend analgesia with lesser side effects is still not clearly defined [16].
The aim of the study is to compare the efficacy of a single low dose of intravenous MgSO4 versus intravenous dexamethasone as adjuvants to ultrasound-guided TAP block for prolongation of postcesarean analgesia.
2. MATERIALS AND METHODS
Our study was performed on 60 pregnant females undergoing elective caesarean sections under general anesthesia who received ultrasound-guided Transversus Abdominis Plane (TAP) block at the end of surgery at the Ain-Shams University Obstetrics and gynaecology hospital, Cairo, Egypt. Randomization was done with the help of an independent statistician using a computer-generated random number table using Microsoft Excel to ensure proper concealment of the study management from the patients and investigators until the release of the final statistical results.
This study was approved by the local ethical Committee at Faculty of Medicine, Ain Shams University Hospital, Cairo, Egypt, under the number of (FAMSU R 59 / 2019) and has been performed in accordance with the ethical standards as in Declaration of Helsinki (1964) and its latter amendments. Written informed consent was obtained from all patients before the study. This research was registered in clinical trial.gov with the following ID (NCT04223128).
Patients were randomly and equally allocated into three groups of twenty patients each. The first group of patients were classified as the Magnesium sulphate group (M), the second group of patients were classified as the dexamethasone group (D) and the third group was classified as the placebo group (C). All the patients, anesthesiologists and the observers who recorded the postoperative data were blinded to the group assignment.
We included pregnant females with singleton full-term pregnancy, with ASA physical status class II, body mass index < 35 kg.m−2, subjected to the elective caesarean section under general anesthesia either upon patients’ request or due to contraindication of neuro-axial anesthesia.
We excluded patients in need of urgent caesarean sections, with multiple gestations, patients with ASA physical status class III-IV, morbid obesity with BMI ≥ 35 kg.m−2 at an initial hospital visit, pre-eclampsia patients who received magnesium sulphate therapy, patients with diabetes mellitus, cardiovascular disease, renal disease, hypermagnesemia, history of analgesic administration or intake during past 24 hours, chronic use of steroids therapy, history of relevant drug allergy, and any possibility of anticipated difficult intubation.
All patients were subjected to a thorough preoperative assessment of their medical history, physical status and laboratory investigations. They were also counseled about the anesthetic management and potential complications of both surgery and anesthesia, and the explanations of the Visual Analogue Scale (VAS) from 0-100 (0 = no pain and 100= worst imaginable pain). All these data were documented. Upon arrival to OR, patients’ identification was confirmed and an 18-gauge intravenous cannula was fixed in all participants in the pre-induction area. Then the injectate of the studied agents’ groups was prepared by one of the researchers in the same volume and color. Participants in group (M) received 50 mg/kg MgSO4 diluted in 100 ml isotonic saline Intravenously (I.V) over 20 minutes prior to induction of general anesthesia by 30 minutes, participants in group (D) received 2 mg Dexamethasone diluted in 100 ml isotonic saline IV over 20 minutes prior to induction of general anesthesia by 30 minutes while participants in group (C) received 100 ml isotonic saline IV (placebo) IV over 20 minutes prior to induction of general anaesthesia by 30 minutes. Induction of general anesthesia to all participants was accomplished by injection of intravenous propofol 2.0 mg/kg, succinylcholine 1.0 mg/Kg, then the oral endotracheal tube was inserted and fixed after confirmation of its place by capnography and auscultation. All patients were monitored throughout the surgery by standard monitors including, electrocardiogram, the pulse oximeter, Non-Invasive Blood Pressure (NIBP), and capnography. Anesthesia was maintained by 2% sevoflurane in oxygen/air mixture 1:1 and muscle relaxation was maintained by atracurium 0.1 mg/kg. After delivery of the fetus and placenta, 2 mg midazolam, 2 µg/kg fentanyl and 10 IU oxytocin diluted in 500 ml ringer solution were administrated and sevoflurane was maintained at 1-2%. After completion of the surgery, TAP block was performed by the same senior anesthesia staff, where the layers of the abdominal wall were identified guided by a superficial high-frequency 45 mm linear array ultrasound probe (13 MHz) as described by McDonnell and colleagues. (4) Using an 18 G Tuohy needle (80 mm Smiths medical Portex®) by the in-plane technique, bilateral injection of twenty millilitre of 0.25% bupivacaine was injected slowly after careful aspiration to avoid inadvertent intravascular injection. The successful injection was indicated by an echo-lucent space between the muscle layers (internal oblique and transversus abdominis).
After TAP block, cessation of the inhaled sevoflurane and reversal of muscle relaxation by 40 µg/kg neostigmine and 10 µg/kg atropine, awake extubation was done and all patients were transferred to the Post-Anesthesia Care Unit (PACU). Upon arrival to PACU, a pulse oximeter and NIBP monitors were attached to the patients. All patients received 1 gm of acetaminophen IV upon arrival to PACU that was continued every 6 hours and 5 mg Nalbuphine IV was given if breakthrough pain (VAS ≥50) developed during the first postoperative day.
All patients were assessed for postoperative pain severity by Visual pain Analogue Score (VAS) as a primary outcome (0 = no pain, 10 - 30 = mild pain, 40 - 60 = moderate pain, 70 - 100 = severe pain) at certain time points started at 30 minutes after recovery, 2, 6, 12 and 24 hours postoperatively. The secondary outcomes included the time intervals till Nalbuphine was firstly requested postoperatively for breakthrough pain (VAS ≥ 50), the total dose of rescue analgesia (Nalbuphine) used within the first 24 hours postoperatively, postoperative hemodynamic variables (heart rate, and mean arterial blood pressure) prior to anaesthesia, post-intubation, post-delivery, post-extubation, and thirty minutes postoperative, time elapsed till patients could ambulate unsupported postoperatively, and the Neonatal APGAR scores were recorded at the 1st and 5th minutes post-delivery.
The primary endpoint to this study was the occurrence of any studied drug related complications (e.g. Convulsions, or arrhythmias), the occurrence of hypoxemia, bleeding, or postoperative atypical hypotension.
2.1. Statistical Analysis
Data were fed to the computer using IBM SPSS software package version 20.0. Qualitative data were described using numbers and percentages. Comparison between different groups regarding categorical variables was tested using the Chi-square test. Quantitative data were described using mean and standard deviation for normally distributed data. For normally distributed data, comparison between two independent populations was done using an independent t-test, while more than two populations were analyzed by F-test (ANOVA). Results of the significance test are quoted as two-tailed probabilities. The significance of the obtained results was judged at 5% level.
2.2. Sample Size Calculation
Our prospective randomized, double-blinded study was performed on sixty consented full-term pregnant women scheduled for elective caesarean section. All participants completed the trial as shown in Fig. (1) . We used PASS II program for sample size calculation, setting alpha error at 5%, power at 100%, and expected SD of 3.5 was obtained from the previous study [ 8 ].
3. RESULTS
The primary aim of this study was the assessment of the effect of administration of MgSO 4 or dexamethasone intravenously on the potency and duration of post-cesarean analgesia achieved by TAP block. There were neither local complications reported related to the TAP block nor systemic complications related to the studied drugs.
The demographic characteristics of the study participants are illustrated in Table 1. There were no statistically significant differences between the three groups regarding maternal age, Body Mass Index (BMI), gestational age, and parity. Furthermore, there were no statistically significant differences between the three groups regarding duration of anaesthesia or duration of surgery (Table 1).
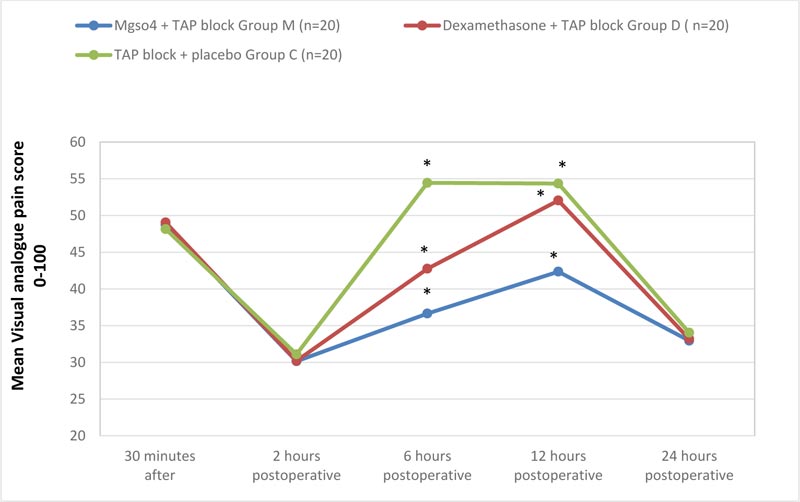
It was noticed that the mean VAS assessed 30 minutes after recovery, 2 and 24 hours postoperatively showed statistically insignificant differences between the three groups (P = 0.281, 0.254, and 0.10 respectively). The comparison of the VAS at 6 and 12 hours postoperatively showed statistically significant lower values in group (M) and group (D) compared to group (C) (P-value at 6 hours was 0.001Vs 0.001 and P-value at 12 hours was 0.001 Vs 0.013 respectively) and also group (M) was significantly lower than group (D) as well (P-value 0.001 and 0.001 respectively) (Fig. 2).
The time interval till the first rescue analgesia (Nalbuphine) needed by the patients (VAS ≥ 50) was significantly longer in group (M) compared to group (D) and group (C) consecutively (P-value of 0.022 and 0.001 respectively). The total dose of rescue analgesia consumed during the first 24 hours postoperatively was significantly lower in groups (M) than group (D), and both groups showed lesser doses compared to group (C) (P-values 0.001, 0.001 and 0.010 respectively). Moreover, the mean time passed till patients could ambulate unaided was significantly shorter in group (M) than in group (D) and both groups showed less time than group (C) when compared separately (P-value 0.001, 0.001 and 0.001, respectively) (Table 2).
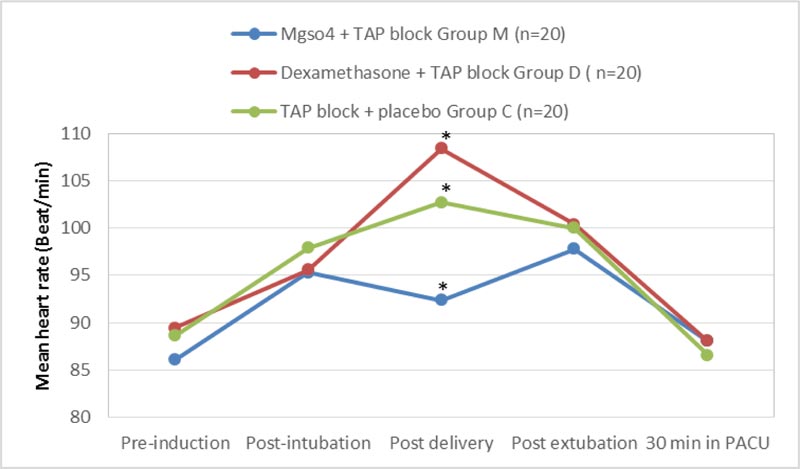
Comparing the maternal hemodynamic variables, we found that the mean heart rate (bpm) was statistically significant and higher in group (D) and (C) compared to group (M) individually in the post-delivery measurements with (P-value 0.001) while it was also significantly higher in group (C) than in group (D) at the same point of measurement (P-value 0.012), but the heart rate during the pre-induction, postintubation, postextubation and 30 minutes immediately postoperative showed no statistical significant differences between the three groups (P-value 0.254, 0.365, 0.298, and 0.398 consecutively) (Fig. 3).
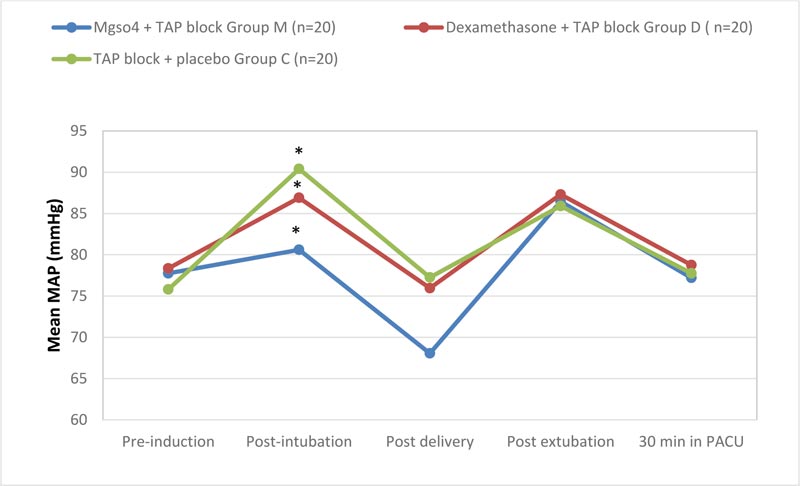
We found that the maternal mean arterial pressure (MAP) (mm Hg) in the post-intubation and post-delivery recordings was significantly lower in group (M) compared to group (D) and group (C) independently (P-value of 0.001and 0.003 respectively), while it was also significantly lower in group (D) compared to group (C) in the post-intubation period only (P-value 0.003). On the other hand, the three groups showed statistically insignificant differences in the pre-induction, post-extubation and 30 minutes postoperative in the PACU (P-value of 0.301, 0.256, and 0.12 respectively) (Fig. 4).
| Variable | Group M (n=20) |
Group D (n=20) |
Group C (n=20) |
F P value |
P1 | P2 | P3 |
|---|---|---|---|---|---|---|---|
|
Age (years) Mean ± S.D. |
29 ± 4.26 | 30.35 ± 4.59 | 28.6 ± 2.96 | 2.31 0.365 |
0.171 | 0.366 | 0.080 |
|
BMI (Kg/m2) Mean ± S.D. |
29.985 ± 1.02 | 30.355 ± 0.96 | 30.54 ± 1.95 | 1.42 0.485 |
0.122 | 0.133 | 0.353 |
|
Gestational age (weeks) Mean ±S.D. |
36.59 ± 0.39 | 36.44 ± 0.26 | 36.56 ± 0.36 | 3.61 0.201 |
0.18 | 0.384 | 0.14 |
|
Duration of surgery (minutes) Mean± S.D. |
45.2 ± 6.78 | 50.2 ± 5.87 | 45.815 ± 8.22 | 4.14 0.107 |
0.091 | 0.399 | 0.080 |
|
Duration of Anesthesia (minutes) Mean ±S.D. |
51.45 ± 5.35 | 54.15 ± 6.87 | 54.8 ± 6.45 | 3.05 0.319 |
0.087 | 0.041 | 0.380 |
P2 comparison between group M and C
P3 comparison between group D and C
F = ANOVA test, p is significant if < 0.05
| Variable | Mgso4 + TAP Block Group M (n=20) |
Dexamethasone + TAP Block Group D (n=20) |
TAP block + Placebo Group C (n=20) |
F P-value |
P1 | P2 | P3 |
|---|---|---|---|---|---|---|---|
|
Time passed till need 1st rescue analgesia (VAS≥5) in hours Mean ± S.D. |
5.43 ± 0.61 | 4.99 ± 0.73 | 4.17 ± 0.64 | 11.2 0.001* |
0.022* | 0.001* | 0.001* |
|
Total dose of rescue analgesia (mg) Mean ± S.D. |
13.29 ± 4.10 | 16.71 ± 4.64 | 21.17 ± 6.71 | 13.52 0.001* |
0.001* | 0.001* | 0.010* |
|
Time needed to ambulate unaided Mean ± S.D. |
4.43 ± 0.36 | 5.38 ± 0.28 | 6.51 ± 0.31 | 15.2 0.001* |
0.001* | 0.001* | 0.001* |
| APGAR Score | |||||||
| 1st Minute Mean ± S.D. |
8.2 ± 0.83 | 8.4 ± 0.50 | 8.25 ± 0.64 | 4.10 0.121 |
0.182 | 0.061 | 0.207 |
| 5th Minute Mean ± S.D. |
9.1 ± 0.72 | 9.4 ± 0.75 | 8.95 ± 0.83 | 2.14 0.362 |
0.214 | 0.366 | 0.080 |
P2 comparison between group M and C
P3 comparison between group D and C
F = ANOVA test, p is significant if < 0.05
4. DISCUSSION
TAP block is considered an efficacious and versatile option in multimodal postoperative analgesia, especially in operations where parietal pain is the major component of postoperative pain. Because of the advantage of TAP blocks which is increased exactness of LA deposition, Ultrasound Guidance standards are beneficial [17].
In this study, we found that a single low dose of both MgSO4 and dexamethasone given preoperatively as adjuvants to ultrasound-guided TAP block enhanced and prolonged the postoperative analgesic effect of local anaesthetic injected compared to the control group as measured by VAS at 6 and 12 hours postoperatively with MgSO4 being statistically superior over dexamethasone. This also was reflected by the time passed till the first rescue analgesia was prescribed to patients and by the total dose of rescue analgesia as well.
Our results are in agreement with Gad et al., who proved that the usage of 200-mg MgSO4 was superior to 8 mg dexamethasone as an adjuvant to bupivacaine injected locally into TAP after total abdominal hysterectomy and concluded that MgSO4 prolonged the duration of analgesia, decreased VAS scores postoperatively, and the number of demands for rescue analgesia more than dexamethasone [18]. Seyed et al., also studied the administration of 50mg / kg IV bolus of MgSO4 to elective cesarean candidates before induction of general anesthesia and concluded that this could reduce acute post-operative pain and have a sparing effect on the morphine demands during the first 24 h [19]. Also, our results are in agreement with a previous research work done by one of the authors where it was observed that administering a low dose of intravenous MgSO4(50 mg/Kg) prior to induction of general anesthesia combined with ultrasound-guided TAP block offered longer postoperative pain-free periods thus reducing total opioid consumption [8].
In contrast to our results, Lysakowski et al., in a systemic randomized trial, discovered no solid proof about the essential advantages of perioperative MgSO4 on postoperative pain severity and necessity of analgesics. Nevertheless, they recommended that further analysis of the role of magnesium as a complement to postoperative analgesia may be helpful, as that drug is inexpensive, relatively harmless, and the biological rationale for its possible anti-nociceptive activity is promising [20].
The dose of dexamethasone used in our study was based on previously published research performed by Dhanger et al, who used low dose IV dexamethasone (2 mg) as an adjuvant with supraclavicular brachial plexus block and they found it significantly effective in prolonging the duration of analgesia and reducing the rescue analgesic requirements without producing any significant side effects [21]. Hence, steroid-induced hyperglycaemia and hypertension were avoided that could occur with high-dose IV regimens [22].
The role of magnesium sulphate in potentiating analgesic effects of local anesthesia injected in the TAP block is not clearly identified but largely attributed to its NMDA receptor antagonism and sympathetic blockage potentials [23].
The mechanism of action of dexamethasone as an analgesic is still debatable which could be explained by the action of dexamethasone on glucocorticoid receptor and changing the function of ion channels especially potassium or local nerve cell acidosis, thereby decreasing the dose of local anesthetic agent required [24]. Moreover, dexamethasone can potentiate local anesthesia by blocking the transmission of nociceptive C-fibres in addition to the suppression of ectopic neural discharge. The duration of postoperative analgesia is prolonged when dexamethasone is used as an adjunct for peripheral nerve blocks [25].
Our results regarding mean maternal hemodynamics recorded intraoperative and 30 minutes postoperatively showed differences between the magnesium sulphate group and the other two groups and these differences could be explained by the well-known fact that MgSo4 blocks the release of catecholamines, hence blunts sympathoadrenal hemodynamic stress response in addition to its direct vasodilation effect, thus attenuating vasopressin-induced vasoconstriction [26]. The APGAR score also showed no differences in the three studied groups denoting the safety of the studied agents on the fetal wellbeing.
4.1. Study Limitations
There were a number of limitations of this research. The first limitation was the relatively small number of cases studied and the second limitation was that the effect of the studied adjuvants was not verified early because the effect of local anesthesia injected is maximally working. The third limitation was the prevalence of neuraxial block as a safer choice for both mother and fetus, which was excluded from our study so as to test for the duration of analgesia provided by the TAP block alone and the effect of both studied adjuvants. These limi tations are advised to be considered in the future
CONCLUSION
This study showed that both intravenous MgSO4 and dexamethasone as adjuvants to TAP block prolong the duration of analgesia, decrease VAS scores postoperatively, and the demands for rescue analgesia with MgSo4 being superior to dexamethasone. However, further studies are required to establish the efficacy of these adjuvants in TAP block.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Local Ethical Committee at the Faculty of Medicine, Ain Shams University Hospital, Cairo, Egypt (FWA 000017585), under the number of (FAMSU R 59 / 2019).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Declaration of Helsinki (1975), as revised in 2013 (http://ethics.iit.edu/ ecodes/node/3931).
CONSENT FOR PUBLICATION
Participants written informed consent was obtained from all patients to be involved in this study.
STANDARDS OF REPORTING
This report follows the protocol established by CONSORT Statement (Clinical Trial Registration Number: NCT04223128).
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the clinical trial.gov, with the following reference ID (NCT04223128).
FUNDING
None.
CONFLICTS OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank all the patients who participated in the study.


