All published articles of this journal are available on ScienceDirect.
Physiological Changes in the Pregnancy and Anesthetic Implication during Labor, Delivery, and Postpartum
Abstract
While providing anaesthetic treatments during emergency surgical procedures, the safety of both the mother and the foetus in utero is the primary goal. Cardiac output, heart rate, and stroke volume decrease to pre-labor values. Maternal blood volume increases during pregnancy, and this involves an increase in plasma volume as well as in red cell and white cell volumes. Oxygen consumption and carbon dioxide production also increases. The respiratory mucous membranes also become vascular, edematous, and friable. Gastric emptying time is significantly slower during labor and hence gastric volume is increased. Furthermore, hepatic transaminases, bilirubin, and LDH are increased slightly in pregnancy. Renal blood flow, glomerular filtration rate and tubular reabsorption of sodium are increased. Hence glycosuria and aminoaciduria may develop in normal gestation. The size of thyroid gland and total T3 and T4 levels are also increased. Hyperplasia of the β-cells occurs. Adrenal cortical hyperplasia leads to increases in both free and total cortisol in pregnancy. Permeability of the blood-brain barrier increases. Altered anatomy and responses to pain and pharmacotherapy occur as pregnancy progresses. The basic aims during the first trimester revolve around avoidance of any drug or technique, which can interfere with proper embryological development. By second trimester, most of the physiological changes have achieved a plateau level and management of anaesthesia becomes relatively safer than in the first or the third trimester. Decision-making in the third trimester becomes a little easier as one can proceed for caesarean section before the major surgery. It is the technical advancements in regional anaesthesia, which has propelled labour analgesia to newer horizons. The provision of a prolonged post-operative pain-free period makes this technique a first choice of many parturients. Eclampsia is one of the most common emergencies encountered by anesthesiologists in our day to day anaesthesia practice.
1. INTRODUCTION
Attending anaesthesiologists have traditionally had a difficult time administering anaesthesia for obstetric and nonobstetric surgery when pregnant. Although there is a dearth of data from underdeveloped countries, statistics from the developed world show that 1-2% of all obstetric patients will require emergency non-obstetric surgery at least once in their lives [1]. Several disorders and associated consequences during pregnancy might lead to a pregnant woman's hospitalization, which may necessitate surgical intervention. Surgical emergencies during pregnancy, such as ovarian cyst torsion, appendicitis, strangulated hernias, severe injuries, and so on, require rapid attention [2]. Although the risk of surgery is similar to that of the general population, anaesthetic management is exceedingly difficult at this time [3]. Safety of both the mother and the foetus in utero is the prime objective when delivering anaesthesia services during these emergency surgical procedures [2]. Despite recent advances in the clinical arena and technology, anaesthesiologists face various difficult challenges in providing safe anaesthetic services. Clinical problems, which include but are not limited to changing demographic characteristics, such as advanced maternal age, obesity, comorbidities, such as diabetes, severe anemia, heart illnesses, and so on, all present an anaesthesiologist with a massive uphill task.
To perform safe anaesthesia, you must have a thorough understanding of the numerous physiological features of pregnancy as well as the pharmacological profiles of various medicines. Both regional (RA) and general anaesthesia (GA) have consequences, some of which are uncommon but can be deadly or permanently incapacitating [4].
General anaesthesia (GA) was once the most often used anaesthetic treatment in obstetrics, including both vaginal and Caesarean deliveries (CS). As the area of obstetric anaesthesia has progressed, neuraxial approaches have largely superseded GA. According to the most recent decennial survey on obstetric anaesthetic practices in the United States, the use of GA for CS has decreased from 35% in 1981 to around 25% in 2011, with the bulk of instances relating to emergency procedures [5]. Approximately 6% of CS patients still require GA and tracheal intubation, according to recent estimates [6]. The greatest feared consequences of GA have generally been failed tracheal intubation and the danger of aspiration and aspiration pneumonitis [7]. In obstetrics, detailed recommendations for the management of problematic intubation have been published [8]. However, when dealing with tracheal extubation and postoperative treatment, it is crucial to remember to take extra precautions.
Over an 18-year period, an analysis of maternal mortality in the state of Michigan (USA) found eight anaesthesia-related deaths; five of these deaths occurred during emergence or in the recovery unit and were caused by airway blockage or hypoventilation [9]. While deaths connected with GA in obstetrics have dropped by approximately 60% from 1979 to 1990 to 1991 to 2002, deaths associated with problematic intubation continue to be reported [10, 11]. CS has a maternal death rate of 2.3 per 100,000 GAs during obstetric GAs, compared with one in 180,000 GAs in the general population; mortality after failure intubation is 1% in parturients. Front of neck airway access is also more common in CS, with 3.4 per 100,000 GAs compared to two per 100,000 GAs in the general population [12]. A failed intubation in an obstetric patient might have serious effects on both the mother and the fetus. A recent study discovered an increased rate of neonatal ICU admissions when the mother's intubation failed [13]. The Confidential Enquirys into Maternal Death reports in the United Kingdom have revealed that a significant reduction in maternal deaths due to GA and airway management is possible; they propose frequent practice and assessment of the skills required to manage difficult intubation in obstetrics [14]. Although aspiration of gastric contents is a rare occurrence (two in 10,000 GAs), identifying risk factors (such as obesity and a history of difficult airways) and adequately preparing (such as fasting and decompression of the stomach before anaesthesia in certain circumstances) are critical in preventing it [12, 15]. When contemplating GA as an anaesthetic option for a pregnant patient, it is important to understand the physiological changes that occur during pregnancy. The changes involving the respiratory, cardiovascular, and gastrointestinal systems are the most important to the anaesthetist. During surgical procedures, four “Hs” are avoided: hypotension, hypoxemia, hypovolemia, and hypothermia. An anaesthesiologist must be cognizant of certain shared critical goals during all three trimesters, taking into account the mother's changing physiology and the functional integrity of uterine blood flow [16, 17].
Concerns about the association between anaesthetic drugs and the possibility of teratogenesis and eventual behavioural damage arise when the developing fetus is exposed to GA. A comprehensive retrospective study based on Swedish birth registries compared almost 5,000 individuals who had surgery during pregnancy, half of whom were under GA, against control subjects. There was no rise in stillbirths or congenital defects, according to their findings [18].
At conventional concentrations, none of the currently used anaesthetics, such as propofol, opioids, neuromuscular blocking agents (NMBAs), and local anaesthetics, have been found to cause teratogenic effects on the fetus at any gestational age [19]. In animal research, neuronal apoptosis has been observed after exposure to anesthetics during the third trimester. The inhibitory action on γ-aminobutyric acid or N-methyl-Daspartate receptors during this phase of strong synaptogenesis has raised questions about the effects of anaesthetic drugs on neurocognitive development in humans. Despite this, there are currently no human studies that show differences in cognitive tests following a brief GA exposure [20]. Because maternal arterial pressure is the primary determinant of fetal perfusion, it is vital to minimize maternal hypotension. When giving GA to a pregnant woman, maintaining adequate maternal oxygenation is equally critical to avoid fetal hypoxemia. When possible, fetal monitoring during nonobstetric surgery or fetal operations gives useful information about the adequacy of perfusion and oxygenation [19]. A plan must be in place prior to surgery to clarify the goal of fetal monitoring (CS delivery or intrauterine fetal resuscitation) and to guarantee that qualified professionals are on hand to speed delivery if fetal HR problems develop. When compared with neuraxial anaesthesia, GA for CS has historically been associated with a somewhat lower base deficit and higher umbilical artery pH following delivery. This is due to the effects of vena cava compression, maternal haemodynamic profile, and vasopressor agent selection rather than the procedure itself [21].
2. METHODS
PubMed, Cochrane Library, Google Scholar, CINAHL, Embase, and PsycINFO databases were used for studies reporting the biochemical function of oxytocin in the regulation of the cardiovascular system from study conception to May 2021. Zotero reference management software for Windows was used to download, organize, review, and cite the articles. Cross-references were also manually searched to identify additional relevant articles. A comprehensive search was performed using the following search terms: “physiological changes in pregnancy”, “anesthetic implication during labor”, “anesthetic implication during delivery”, “anesthetic implication during labor, delivery, and postpartum,” and “anesthetic implications in women with eclampsia”. Boolean operators like “AND” and “OR” were used to combine search terms.
3. RESULT/DISCUSSION/ ON THE PHYSIOLOGICAL CHANGES IN THE PREGNANCY AND THEIR ASSOCIATED ANESTHETIC IMPLICATIONS
3.1. Changes Related to Cardiovascular System
Early in pregnancy, physiological changes occur, resulting in overall hyperdynamic circulation. As early as 8 weeks of pregnancy, peripheral vasodilation and a decrease in systemic vascular resistance (SVR) occur as a result of elevated levels of oestrogen and progesterone [22, 23]. Because the uteroplacental circulation lacks autoregulation, cardiac output (CO) must rise to maintain blood pressure (CO × SVR) [24]. If arterial pressure (AP) is to be maintained, which is necessary for a well-functioning uteroplacental unit, cardiac output (CO) must be increased. During pregnancy, cardiac output increases by 30-40%, with the largest increase occurring around 24 weeks of pregnancy [25]. This is accomplished initially by increasing stroke volume (SV), but as the pregnancy proceeds, the increase in SV plateaus, and the heart rate rises. This rise in CO is achieved in early pregnancy by increasing the heart rate (HR) by 15-25 percent, followed by a 20-30 percent increase in stroke volume (SV) [26]. Heart rate rises quickly (before the end of the first month of pregnancy) and then plateaus at 10-15 beats per minute by 28-32 weeks of pregnancy. On the other hand, stroke volume increases by the mid-first trimester and continues to rise during the second trimester, with the increase in SV due to an increase in both end-diastolic volume (EDV) and ventricular muscle wall mass. The increased blood volume, which increases from 6 to 8 weeks gestation to a maximum amount at 32 weeks, causes increased preload and EDV. When compared to a nonpregnant person, there is a total increase in blood volume of up to 2000 ml. As a result, the pregnant patient is able to adjust for blood loss effectively [27]. Beginning at 6 to 8 weeks of pregnancy, blood volume increases to roughly 20% by the mid-third trimester [28]. The renin-angiotensin system is activated by a wide pulse pressure and low mean arterial pressure, which causes salt and water retention. This causes a 40-50 percent rise in plasma volume. Furthermore, echocardiography showed increased end-diastolic chamber size and total left ventricular wall thickness but no change in end-systolic volume, indicating an increase in ejection fraction.
| S.NO | Changes in cardiac auscultation | Changes in electrocardiogram |
| 1. | Loud S1 | Shortened PR and QT interval |
| 2. | Ejection systolic murmur | Shifted QRS axis |
| 3. | S3 and S4 (No clinical significance) | Rightwards (1st trimester) |
| Leftwards (3rd trimester) | ||
| ST segment depression | ||
| Isoelectric low voltage T waves in both precordial and limb leads |
The heart is also shifted cephalad and laterally due to anatomical alterations caused by a gravid uterus. In both cephalad and lateral directions, the heart is physically dilated and displaced. A healthy pregnancy ECG with 15-200 degrees of left axis deviation and inverted T waves in the lateral lead and lead III can simulate left ventricular hypertrophy and other structural diseases [29]. Because of the gravid uterus' gradual elevation of the diaphragm, the heart is shifted to the left and upward throughout pregnancy. Sinus tachycardia or benign dysrhythmias, depressed ST segments and flattened T waves, left axis deviation, and left ventricular hypertrophy can all be seen on the electrocardiogram of normal parturients. Auscultation typically reveals a tricuspid or mitral regurgitation systolic murmur, as well as a third or fourth heart sound. Cardiac output, heart rate, and stroke volume recover to prelabor levels 24-72 hours after birth and to nonpregnant levels 6-8 weeks later [30]. (Table 1) lists the changes in cardiac auscultation and electrocardiogram [31].
The uterus, kidneys, and skin get the majority of the rise in CO, which is used to feed nutrition to the foetus, eliminate maternal and foetal waste products, and aid in maternal temperature control, respectively [32-34]. However, the cardiac output varies depending on the size of the uterus and the position of the mother at the time of measurement. The pressures in the femoral venous and inferior vena caval are significantly higher in normal pregnant women. Atrial filling is maintained by collateral vessels, although veins, notably the epidural venous (Batson's) plexus, are engorged [22]. Due to myocardial remodeling during pregnancy, filling pressures (CVP, pulmonary capillary wedge pressure, LV end-diastolic pressure) stay unchanged despite enlarged heart dimensions [35].
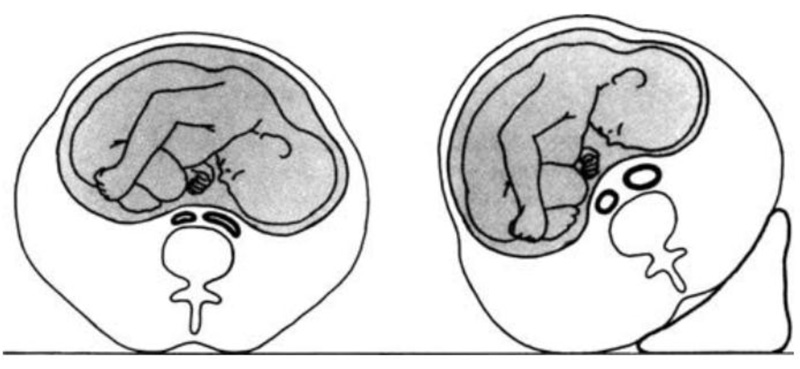
While the pregnant lady is in the supine posture, the enlarged gravid uterus might produce aortocaval compression and diminished ventricular filling, resulting in an underestimate of cardiac performance (Fig. 1). The iliac veins combine to produce the inferior vena cava at a level that corresponds to the L4/5 interspace anatomically. Inferior vena cava compression may occur after the uterus reaches this level. The mechanical consequences of the expanding uterus can produce compression of both the inferior vena cava and the descending aorta in the supine position by the time the uterus hits the level of the umbilicus, which corresponds to 20 weeks in a singleton pregnancy. The combination of these factors results in a lower venous return and a lower CO level [36]. When turning from the lateral to the supine position at 38-40 weeks gestational age, CO drops by 25-30 percent.
Because the uteroplacental circulation lacks autoregulation, this results in lower uterine blood flow and placental perfusion. Additionally, systemic vascular resistance is reduced by roughly 20%. During a normal pregnancy, blood pressure does not rise, and systolic and diastolic blood pressures decrease by about 8% and 20%, respectively [35]. The reduction in blood pressure reported despite an increase in cardiac output could be due to pregnancy hormones (estradiol and progesterone), prostacyclin, and nitric oxide. As a result of aortocaval compression, maternal hypotension and consequent foetal deterioration (acidaemia) might occur [24]. Increased sympathetic tone causes vasoconstriction and tachycardia, and blood flow from the lower limbs is diverted through the vertebral plexus and azygos veins to reach the right heart are among the maternal compensatory mechanisms for aortocaval compression. These compensatory processes may be insufficient in many parturients to maintain blood pressure in the supine posture, resulting in supine hypotension (or aortocaval compression syndrome) [37, 38]. Pallor, transitory tachycardia followed by bradycardia, perspiration, nausea, hypotension, and dizziness in the supine posture are all symptoms that are eased by shifting to the side. It can cause unconsciousness or maternal death in its most severe form [24]. Even if the mother is asymptomatic, the mother's uterine blood flow may be hampered [36].
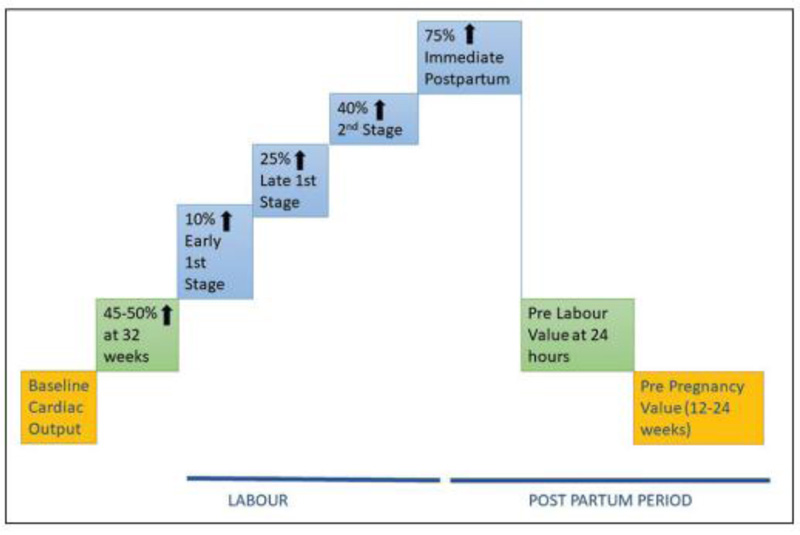
During labor, however, cardiac output increases even more, up to 50% greater than prelabor levels, despite the fact that adequate analgesia can mitigate some of this increase. Cardiac output rises to a maximum of 80 percent above prelabor levels and around 150 percent above nonpregnant levels in the early postpartum period. The higher cardiac output is maintained by an increase in stroke volume as well as heart rate. Despite the higher CO, there is a transitory drop in AP, resulting in wider pulse pressure and lower mean arterial pressure. This activates the renin-angiotensin system, causing water and salt retention and, as a result, an increase in plasma volume [36]. The changes in CO during labour and the postpartum period are summarized in (Fig. 2) [39, 40].
3.2. Clinical Implications Related Anaesthesia
The apical impulse is moved laterally and cephalad to the fourth intercostal space due to structural alterations. Increased blood volume provides some reserve for normal blood loss during delivery (300-500 mL for vaginal delivery, 600-1000 mL for caesarean delivery) and peripartum haemorrhage. However, because of this increase in blood volume, pregnant women may not show signs and symptoms of hypovolemia until roughly 1500 ml of blood has been lost [24]. More than 1500 ml of blood may have been lost by the time the usual symptoms and signs of hypovolaemia, such as tachycardia, hypotension, and oliguria, appear [27].
Venepuncture and i.v. cannulation are much easier with peripheral vasodilation. During epidural anaesthesia, epidural veins enlarge, increasing the risk of intravascular injection—also known as a “bloody tap.” This is compounded by the fact that 500 ml of blood is discharged from the uterus into the maternal circulation during uterine contractions. In the event of hypotension, greater doses of vasopressors such as phenylephrine are necessary due to the downregulation of adrenergic receptors. I.V. and inhalation anesthetics, which cause a decrease in SV and CO, as well as neuraxial block, which causes sympathetic block, all enhance the risk of supine hypotension. Supine hypotensive syndrome can be aggravated by a reduction in SV and CO during general anaesthesia (GA) and sympathetic suppression during neuraxial anaesthesia. As a result, the supine position should be avoided or the uterus displaced laterally with a wedge under the hip. Once the foetal head is engaged, the negative effects of aortocaval compression are reduced. The Oxford position has been found to have better haemodynamic stability, more reproducible block height, and prevented aortocaval compression during neuraxial anaesthesia. It is a modified lateral position with an upward slope in the thoracic region, with no supine position allowed until surgery starts. If the left lateral tilt is used in the event of cardiac arrest and cardiopulmonary resuscitation, it must be done with the parturient on a firm, flat surface to allow for effective chest compression. If this is not possible, the supine position with manual uterine displacement must be used [41]. Pain and auto-transfusion add to the cardiac workload during labor and delivery. In the second stage of labor and the early postpartum period, parturients with a low cardiac reserve are at a higher risk of ventricular failure and pulmonary oedema. Early in labor, regional analgesia should be used and then gradually reduced after delivery. It is also worth noting that incipient cardiac disease is a leading cause of maternal death in the United Kingdom. Many of the initial symptoms and signs are ‘soft' and are mistakenly attributed to pregnancy's physiological changes. Maintain a high level of suspicion, and if there is any doubt, additional investigations and cardiology referrals should be considered [42]. Pregnant women with valvular heart disease (e.g., aortic or mitral stenosis) or coronary artery disease may not tolerate increased cardiac output. Myocardial decompensation can occur as early as 24 weeks of pregnancy, during labor, and especially soon after delivery. The risk of intravascular catheter placement in pregnant women is increased by engorgement of the epidural venous plexus; the direct connection of the azygos system to the heart and brain also increases the risk of local anesthetic cardiovascular and central nervous system toxicity [43].
3.3. Changes Related to Hematological System
During pregnancy, the volume of the mother's blood increases, which includes an increase in plasma, red blood cells, and white blood cells [44]. While the increase in plasma volume is around 40-50%, the increase in red blood cell mass is only around 15-20%. Dilutional physiological anemia (normal hemoglobin 12 g/dL; hematocrit 35) is caused by a discrepancy in the increase in plasma volume and red cell mass during pregnancy [28]. Blood viscosity is reduced by about 20% as a result of this hemodilution. A lower haematocrit lowers blood viscosity and blood flow resistance in the uteroplacental circulation [24]. The exact mechanism of this increase in plasma volume, on the other hand, is unknown. Several mediators are considered involved, including renin-angiotensin-aldosterone, atrial natriuretic peptide, estrogen, progesterone, and nitric oxide. The most likely explanation is that the increase is due to a “underfill” state caused by initial vasodilation, which causes fluid retention by stimulating hormones like renin, angiotensin, and aldosterone [45]. Alternatively, some have proposed an “overfill” state characterized by an early increase in sodium retention (due to an increase in mineralcorticoids) followed by fluid retention, resulting in an increase in blood volume and then vasodilation. As uterine contractions squeeze blood out of the intervillious space and into the central circulation during labor, blood volume increases even more. After delivery, the uterus involutes and placental circulation ceases, resulting in a blood autotransfusion of approximately 500 mL. The leukocyte count gradually rises to around 15,000 cells per millimeter [32]. This may increase during labor and delivery, but it is not a sign of developing sepsis. Polymorphonuclear cells, which are dysfunctional, play a major role in this increase. Because sepsis is a state of altered immune competence (allowing for paternal antigens in fetoplacental tissue), pregnant patients are predisposed to developing it [36]. This explains why infections are more severe, but it does not mean that parturients are more susceptible to infections [46]. The production of autoantibodies and the levels of immunoglobulins A, G, and M are unaffected [24].
All clotting factors (except XI and XIII) and fibrinogen increase during pregnancy, while natural anticoagulants decrease and fibrinolytic activity decreases [47]. Standard clotting screens do not usually reflect the changes that occur. The overall effect of these changes is variable, but prospective observations have shown that platelet count decreases as pregnancy progresses, with 7.6% of women having a count less than 150,000 and 1% having a count less than 100,000 at term [48]. Platelet production and thrombopoietin levels are also increased [49], and platelet aggregation measured in vitro is likewise increased; indices of platelet destruction are also increased. Although platelet production increases, the count does not increase due to increased destruction and haemodilution. The platelet count, on the other hand, drops as a result of increased destruction and haemodilution, which occurs at its peak in the third trimester. Platelet count decreases (90,000-100,000) in a minority of women, which is normal (gestational thrombocytopaenia) and resolves in the postpartum period [50]. With an increased risk of thromboembolism during pregnancy (10 times) and postpartum, the coagulation and fibrinolytic pathways are altered (25 times). In a normal pregnancy, endogenous anticoagulants such as protein S are reduced, and activated protein C resistance is acquired throughout pregnancy, adding to the prothrombotic condition. Placental-derived plasminogen activator inhibitor (PAI) impairs fibrinolysis throughout normal pregnancy, but it returns to normal when the placenta is delivered [51]. As a result of the physiologic changes, pregnancy becomes hypercoagulable [52]. Prothrombin time and activated partial thromboplastin time have both been reduced by 20%. There are also higher levels of fibrin breakdown products and plasminogen [53]. The hypercoagulable state lasts for 5-7 days after delivery, with a higher risk of thrombotic problems, before reverting to baseline by 2 weeks.
3.4. Clinical Implications Related Anaesthesia
Increased blood volume and improved coagulation serve two key functions: (1) they meet the increased circulatory needs of the expanding uterus, fetus, and placenta, and (2) they protect the parturient from bleeding during birth [43]. When deciding on fluid and blood replacement in the peripartum phase, anesthesiologists should take into account the increased blood volume. Parturients become hypercoagulable as their pregnancy advances, putting them at risk for thromboembolism.
Blood volume gradually returns to normal over 8 weeks after a fast mobilization and diuresis of some fluid in the first few postpartum days [43]. During the antenatal period and again upon admission to the hospital, all pregnant women should have their thromboembolic risk assessed and appropriate thromboprophylaxis administered. Because low molecular weight heparins (LMWHs) are increasingly being utilized in the antenatal period, it is critical that the anesthesiologist is informed of this, as well as the time since the last dose, as regional blocks should not be performed within 12 hours after a prophylactic LMWH dose [54]. In an otherwise healthy parturient, platelet counts are usually within normal ranges, and the results of a platelet count are not required prior to regional anaesthesia.
The platelet count can drop fast in severe preeclampsia, putting the parturient in danger of an epidural haematoma. A recent platelet count (within 6 hours) should be conducted both before sitting an epidural and before withdrawing the epidural catheter for the group of patients whose condition is considered severe enough for the Collaborative Eclampsia Trial regimen of magnesium sulphate [55]. There is no one platelet level that predicts the development of neuraxial haematoma; platelet number and function are both relevant. However, testing for both platelet count and function is recommended (aggregability). In an emergency, laboratory testing might take a long time, therefore, thromboelastography can help assess the entire coagulation process (initial fibrin plug creation, platelet aggregation, clot strengthening, and fibrinolysis) [56]. TEG (thromboelastography) is a point-of-care bedside test that determines global haemostatic function. TEG monitors the entire coagulation process, from fibrin production to platelet interaction, clot strengthening, and fibrinolysis. Platelet function is shown by the maximum amplitude (MA). In the presence of thrombocytopenia and aberrant TEG, most anaesthetists will not deliver regional anaesthesia [55].
3.5. Changes in the Respiratory System
As early as the fourth week of pregnancy, changes in respiratory parameters begin. To meet the metabolic demands of the mother and fetus, anatomical and physiological changes occur. Oxygen consumption and carbon dioxide generation rise by about 60% compared to prepregnancy levels. By the second trimester, there has been a 45 percent increase in minute ventilation due to an early increase in tidal volume [57]. At term, minute ventilation is raised by around 50% over nonpregnant levels. The increase in minute ventilation is mainly due to an increase in tidal volume (40%) and, to a lesser extent, an increase in the respiratory rate (15%) [58]. There is a corresponding increase in alveolar ventilation.
Progesterone acts as a respiratory stimulant, making chemoreceptors more sensitive to carbon dioxide (CO2) [59]. PaCO2 decreases to 30-32 mmHg in the first trimester and remains in this range throughout pregnancy because of increased CO2 production (about 300 ml/min) and higher MV. End-tidal CO2 and PaCO2 do not have a gradient. The increase in ventilation leads to decreased arterial carbon dioxide tension with an average PaCO2 of 4 kPa at term. In active labor, this can be reduced even more. This would normally result in respiratory alkalosis, which is somewhat mitigated by lowering serum bicarbonate levels to around 20-21 mEq/L and a pH of 7.42-7.44. Although renal bicarbonate excretion keeps arterial pH stable, PCO2 drops to 32-35 mmHg at term. Even before a rise in metabolic rate, increased progesterone concentrations during pregnancy are likely to drive increased respiration [60].
Early in pregnancy, PaO2 rises due to a decrease in PCO2. Progesterone is the primary force behind this, as it reduces the respiratory center's carbon dioxide response threshold. PaO2 rises during pregnancy as a result of increased alveolar ventilation; however, after mid-gestation, PaO2 declines in the supine position when functional residual capacity (FRC) falls below the closing capacity, resulting in airway closure during tidal volume breathing. The diaphragm is pressed in a cephalad direction as the uterus swells. In the upright posture, the functional residual capacity (FRC) is reduced by 20%, while in the supine position, it is reduced by up to 30% (Table 2). At the end of the term, the functional residual capacity, expiratory reserve volume, and residual volume are all reduced (Fig. 3). These alterations are due to the diaphragm's cephalad displacement caused by the big gravid uterus. Because of the rise in tidal volume and inspiratory reserve volume, the inhalatory capacity increases slightly. The vital ability remains the same. Because of the increase in chest circumference, total lung capacity is only slightly diminished. The closing capacity (CC) remains unchanged, but the decrease in FRC contributes to an earlier desaturation by allowing lung volume to go below CC more quickly. The rightward shift in the maternal oxygen dissociation curve increases oxygen delivery to the foetus, and the P50 value rises at term (30 vs. 26 mmHg). The P50 of fetal haemoglobin is around 18 mmHg, indicating that it has a stronger affinity for oxygen. Despite the diaphragm cephalad displacement, diaphragm excursion during breathing increases by 2 cm while chest wall excursion decreases.
Flow volume loops show no change as a result [61]. Due to increased metabolic demand, MV increases by 70-200%, PaCO2 drops to 10-15 mmHg, and oxygen consumption rises by 40-75% during labor. By 6-8 weeks after delivery, MV, TV, and oxygen consumption return to prepregnancy levels. Within 6-12 weeks after delivery, all other respiratory indicators revert to prepregnancy levels. Following the reduction in uterine size, FRC alterations return to normal 1-2 weeks postpartum.

Pregnancy causes anatomical changes as well. The mucous membranes of the lungs become vascular, edematous, and friable. Capillary engorgement of the nasal, oropharyngeal, and laryngeal mucosa occurs as a result of oestrogen's impact. Anteroposterior and transverse diameters of the chest wall grow by 2 cm each, resulting in a 5-7 cm rise in circumference [23]. As the tidal volume rises, alveolar ventilation rises dramatically, with no change in the ratio of dead space to tidal volume (VD/VT). During pregnancy and labor, the voice may deepen, and the Mallampati score rises gradually [62].
| S. No | Parameter | Change During Pregnancy |
| 1. | Expiratory Reserve Volume | Decreased by 25% |
| 2. | Residual Volume | Decreased by 15% |
| 3. | Functional Residual Capacity | Decreased by 20% |
| 4. | Tidal Volume | Increased by 45% |
| 5. | Inspiratory Reserve Volume | Increased by 5% |
| 6. | Inspiratory Capacity | Increased by 15% |
| 7. | Vital Capacity | No Change |
| 8. | Total Lung Capacity | Decreased by 5% |
| 9. | FEV1 | No Change |
| 10. | FEV1/FVC | No Change |
| 11. | Closing Capacity | No Change |
3.6. Clinical Implications Related Anaesthesia
The majority of pregnant women feel physiological breathlessness, but it can also be the presenting symptom of serious underlying respiratory or heart problems. Breathlessness that comes on suddenly or is accompanied by chest pain, orthopnoea, and paroxysmal nocturnal dyspnea are all signs of a more serious condition [36]. Despite appropriate pre-oxygenation, a lower FRC and higher oxygen demand can result in fast desaturation during apnoea [63]. Reduced FRC also reduces denitrogenation time and speeds up the uptake of inhaled anesthetics. Gas exchange at the alveolar level is facilitated by greater minute breathing and a lower FRC, resulting in a faster rate of inhalation agent uptake and changes in depth of anaesthesia. When ventilating a parturient, keep in mind the lower levels as well as the comparable gradients between end-tidal CO2 and PaCO2. The lack of gradient is attributed to a reduction in alveolar dead space (increased blood perfusion from an increase in maternal CO). Hyperventilation should be avoided since it can lead to respiratory alkalosis and a left shift in the oxygen dissociation curve, which means less oxygen gets to the fetus. Because of decreased uteroplacental perfusion due to uterine vasoconstriction and tilting of the maternal oxygen dissociation curve to the left, maternal alkalosis along with decreased PaCO2 values as a result of hyperventilation as a result of labor pain can cause fetal acidosis. Uncontrolled mother discomfort during labor can raise metabolic needs even further, resulting in an increase in maternal lactate levels, indicating that oxygen demands are greater than supply. In the absence of pain medication, minute volume increases even more during labor and PCO2 may drop to 17 mmHg. Despite a better response to hypoxic ventilator drive, a vulnerable parturient's higher oxygen demand cannot be met without the use of supplemental oxygen. Although opioids help to mitigate this alteration, epidural analgesia is advantageous since it lowers metabolic demands during labor [64]. Even in the context of good regional analgesia, maternal expulsive attempts boost breathing in the second stage [65, 66].
Anatomically, capillary engorgement causes swelling and friability of the nasopharyngeal and oropharyngeal tissues. Because of the increased risk of epistaxis during pregnancy, the nasopharyngeal approach should be avoided due to increased edema, vascularity, and friability of the mucous membrane. The airway of the parturient can become obstructed, making tracheal intubation more difficult. The Mallampati class is still widely used to predict intubation, but it has been demonstrated to have a low positive predictive value. When combined with other difficult airway predictors, it can be quite beneficial. During pregnancy, and especially during labor, the Mallampati categorization deteriorates [62]. The Mallampati score can alter depending on the stage of labor, with the greatest increase in Mallampati 3 and 4 grades occurring between the first and second phases [67]. This, combined with larger breasts and rising obesity rates among pregnant women, can make laryngoscopy more challenging. In tough situations, laryngoscopes with short handles, smaller diameter endotracheal tubes, and a ramp position at the head end may be required.
4. CHANGES IN THE GASTROINTESTINAL SYSTEM
The gastrointestinal (GI) system's secretory and absorptive activities are unaffected, but motility is. The stomach is increasingly shifted upwards by the gravid uterus as the pregnancy advances, resulting in an altered axis and greater intragastric pressure. The lower esophageal sphincter is displaced and disrupted as the uterus grows larger. In the majority of cases, the intra-abdominal part of the oesophagus is displaced into the thorax. Progesterone, on the other hand, induces the lower oesophageal sphincter (LOS) to relax [68]. These anatomic and hormonal changes promote a decrease in LOS tone, which manifests as pregnancy-related gastro-oesophageal reflux illness (up to 80 percent at term). Heartburn is caused by an increase in stomach pressure caused by mechanical compression. Despite the fact that this symptom is common, overall acid production is reduced (although placental production of gastrin increases the total concentration of this hormone).
The majority of pregnant women (80%) experience nausea and vomiting [24]. Acetaminophen absorption, ultrasonography, dyedilution, and radiography methods all show that gastric emptying is normal throughout pregnancy. Because progesterone inhibits GI contractile activity, oesophageal peristalsis and intestinal transit slow down, causing constipation. Although no difference in stomach emptying time has been seen (even in obese females), it is slowed during labor and the postpartum period [69]. Studies of stomach pH and volume in pregnant and nonpregnant women reveal no differences in the proportion of women who meet “at risk” criteria for pulmonary aspiration of gastric contents (pH 2.5, volume >25 ml)(22) [70]. This pattern is substantially altered by labor. During labor, the gastric emptying time is much slower, resulting in an increase in gastric capacity. Opioids, in any form, will lengthen the time it takes for the stomach to empty. Solid food can be found in the stomachs of laboring women even after 18 hours of fasting, according to studies [71]. On the first postpartum day, gastric emptying is aberrant, but on the second day, it returns to normal. Within 1-2 days after delivery, the alterations in the GI system return to normal [69].
Due to increased oestrogen levels, spider nevi and palmar erythema, both markers of liver disease, can be physiologically seen during pregnancy [72]. Despite an increase in CO, there is no corresponding increase in hepatic blood flow. More than half of pregnant women have oesophageal varices as a result of an increase in splanchnic, portal, and oesophageal venous pressure, which recover quickly postpartum [73]. Serum albumin, transaminases (AST and ALT), and bilirubin levels are all lower in pregnant women than in nonpregnant women on conventional liver function tests. Hepatic transaminases, bilirubin, and LDH, on the other hand, are modestly elevated during pregnancy.
Dilution owing to increased plasma volume produces a 60 percent drop in serum albumin concentration [74]. Serum alkaline phosphatase (ALP) levels may be physiologically high (2-4 fold), especially in the third trimester, due to placental synthesis of ALP at the brush border membranes of the syncytiotrophoblast rather than hepatic alterations. HELLP syndrome (haemolysis, increased liver enzymes, and low platelets) in the parturient, intrahepatic cholestasis, cancer, and primary liver or bone disease can all induce elevated levels [75].
Plasma cholinesterase activity decreases from the 10th week of pregnancy through the third postpartum day, when levels are up to 33% lower than nonpregnant levels. This is due in part to the haemodilution impact and in part to the liver's diminished synthesis. The activity of serum cholinesterase is lowered by 24% before delivery and reaches a nadir (33% reduction) on the third postpartum day [65]. Gallbladder function and emptying are hampered during pregnancy, and research suggests that pregnant women are more likely to develop gallstones.
4.1. Clinical Implications Related Anaesthesia
Gastric emptying is not delayed in the non-laboring term parturient. The fasting guidelines, on the other hand, do not need to be changed. Regardless of when they last ate, pregnant women in labor should always be regarded as having a full stomach. Gastric emptying is delayed in the laboring patient, who may be experiencing cumulative effects from anxiety and labor pain. Women who have taken opiates by any route, including epidural or subarachnoid, experience a delay in gastric emptying [76]. As a result of the increased intra-abdominal pressure and reduced LOS tone, there is a higher risk of aspiration of gastric contents. During general anesthesia and intubation, the risk is raised. When general anesthesia is unavoidable, conventional precautions (quick sequence induction and endotracheal intubation) should be used instead [43]. Preference for neuraxial procedures and the use of aspiration prophylaxis are two more essential stages in prevention. Before a cesarean section or the induction of regional anesthesia, it is critical to use a nonparticulate antacid on a regular basis and to allow for optimal mixing of the antacid and stomach contents. Aspiration causing Mendelson's syndrome (acid aspiration generating an inflammatory response in the lung parenchyma resulting in chemical pneumonia) is becoming less common. Pregnant women who are not in labor or have no other risk factors for aspiration may not need to be treated. A moderate dose of clear fluid is advised in simple labor [77]. Oral intake during normal labor, on the other hand, is a contentious topic. The safest and most successful technique for preventing aspiration is to employ a regional anaesthetic [36]. Antacid premedication should be recommended for all patients starting at 16 weeks of pregnancy. When histamine H2-receptor antagonists are combined with sodium citrate, the mean pH rises and the percentage of patients with a gastric pH < 2.5, which is essential for chemical pneumonitis, falls. If general anaesthesia is required, a rapid sequence induction approach with cricoid pressure and a cuffed tracheal tube should be employed following preoxygenation. Gastrointestinal effects return to prepregnancy levels 24-48 hours after delivery.
Despite a decrease in plasma cholinesterase, a single dosage of succinylcholine does not have a clinically meaningful impact on prolonging. This is most likely owing to increased distribution volume and decreased sensitivity [24]. However, to increase sensitivity to succinylcholine, the activity must be reduced by 50%; therefore, medication doses must be modified.
Around 11% of postpartum women have clinically inadequate activity, which manifests as an increased reaction to typical succinylcholine dosages. Even with this decreased activity, when general anesthesia is required, regular succinylcholine dosing for intubation is suggested, although the use of a peripheral nerve stimulator seems reasonable. However, if the parturient has HELLP syndrome, caution should be exercised because more than 60% of these individuals have pseudocholinesterase activity below the normal range [78].
5. CHANGES IN THE RENAL SYSTEM
By the second trimester, there has been a 50% rise in renal blood flow, resulting in an increase in renal size and an increased glomerular filtration rate from 100 to 150 ml/min. Because of the increased renal plasma flow during pregnancy, the glomerular filtration rate rises [79]. Renal blood flow and glomerular filtration rate (GFR) rise, although histology and the number of nephrons remain unchanged [34]. The concentrations of plasma blood urea nitrogen (BUN) and creatinine are reduced by around 40%-50% when the filtration rate is increased to roughly 8-9 mg/dL and 0.5-0.6 mg/dL, respectively [24]. As a result, blood urea and creatinine levels are lower in pregnant women than in nonpregnant women; thus, values at the upper end of the “normal range” indicate impaired renal function during pregnancy. By the sixth postpartum week, the glomerular filtration rate and BUN concentration had gradually returned to pre-pregnancy levels [43].
Sodium reabsorption in the tubules is enhanced. The enhanced GFR, however, surpasses the capacity of reabsorption within the proximal tubules, resulting in higher glucose and protein levels in the urine. Urinary proteins have a maximum ‘normal limit' of 300 mg per day, which is double what is considered normal in the non-pregnant condition [80]. As a result, glycosuria and aminoaciduria can occur throughout normal pregnancy. Urinary protein excretion increases with multiple pregnancies (150-200 mg/day at term vs. roughly 100 mg/day prepregnancy). Pregnancy-induced physiologic hypoalbuminemia might cause a decrease in the anion gap (from 10.7 to 8.5). In the absence of diabetes or renal illness, reduced tubular function and decreased fractional reabsorption can cause glucose and aminoaciduria [23].
The renal pelvis and calyces are likewise dilated, and peristalsis is reduced due to progesterone and mechanical compression of the ureters. Physiological diuresis develops between the second and fifth days after delivery [43]. Due to hormonal influences, external pressure, and intrinsic alterations in the ureteral wall, hydronephrosis and hydroureter are frequent during pregnancy.
Urinary incontinence, nocturia, and urinary tract infections are all common problems [24]. Due to decreased vascular receptor expression, the vascular response to vasopressors (angiotensin II, norepinephrine, and antidiuretic hormone) is also reduced. During pregnancy, nitric oxide production increases, resulting in systemic and renal vasodilation. During pregnancy, normal plasma osmolality decreases (approximately 270 mosmol/kg vs. 275-290 mosmol/kg prepregnancy) and plasma sodium concentration decreases proportionally (4-5 meq/l below prepregnancy norms). Thirst and pituitary production of Antidiuretic Hormone (ADH), which are normal physiological reactions to osmolality fluctuations, are unaffected. By 4-6 weeks after delivery, all alterations in the renal system have returned to their prepregnancy status.
5.1. Clinical Implications Related Anesthesia
In parturients, normal nonpregnant BUN and Cr levels indicate impaired kidney function. Urinary protein excretion of more than 300 mg per day should be investigated further [81]. Because the normal range of serum creatinine in pregnant women is lower, a little increase in readings suggests a bigger loss in renal function. Low albumin levels cause highly protein-bound medicines, including digoxin, midazolam, thiopentone sodium, and phenytoin, to become more free [23]. Aldosterone's effects are heightened, resulting in increased water absorption and a rise in the volume of distribution and elimination half-life of certain medications, such as thiopental.
Although the primary renal disease is uncommon, renal function impairment is evident in more frequent pregnant diseases such as preeclampsia. Renal impairment necessitates a cautious anesthetic strategy that includes careful hydration management, diabetes control, and medication dose and protocol adjustments [24].
6. CHANGES IN THE ENDOCRINE SYSTEM
Because of follicular hyperplasia and enhanced vascularity, the thyroid gland can grow by about 20% during pregnancy. Total T3 and T4 levels rise by 50% as a result of an increase in thyroid-binding globulin caused by oestrogen, while free T3 and T4 levels remain the same [24]. Thyroid stimulating hormone levels drop during the first trimester of pregnancy but then rise throughout the remainder of the pregnancy. Subclinical hypo- and hyperthyroidism can develop and are not linked to negative consequences [82, 83]. Thyroid stimulating receptors (TSH) in the thyroid gland are stimulated by increasing amounts of human chorionic gonadotropin (Hcg) because the subunits of TSH and hcg are similar. This causes hyperthyroidism and hyperemesis gravidarum in the short term.
When compared to prepregnancy state, human placental lactogen induces diminished tissue sensitivity to insulin and consequently greater blood glucose levels following carbohydrate-rich meals during pregnancy. During hunger, pregnant women suffer hypoglycemia and ketoacidosis quickly. Fasting blood sugar levels are lower in pregnant women than in nonpregnant women, but placental lactogen's effects may reduce glucose tolerance, resulting in a moderate diabetogenic state. This can sometimes lead to gestational diabetes. Pregnancy is a diabetogenic state with increased insulin production and secretion as a compensatory mechanism. In the islets of Langerhans, hyperplasia of the β-cells occurs.
Fetal insulin levels are unrelated to maternal insulin secretion, although they are affected by the maternal glucose load and hence the amount of glucose accessible for placental transfer. Maternal glucose management issues can lead to fetal macrosomia and neonatal hypoglycemia after delivery. After the placenta is delivered, glucose responses quickly return to normal. During pregnancy, placental lactogen and dopamine produce hyperprolactinemia. The pituitary gland stores 30 percent more oxytocin, which is released during labor and shortly after birth [84]. To prevent premature labor, the oxytocin response to stress is reduced throughout pregnancy. In pregnancy, adrenal cortical hyperplasia causes an increase in both free and total cortisol.
6.1. Clinical Implications Related Anaesthesia
Airway evaluation, ECG, invasive cardiovascular monitoring, treatment of hydration, electrolyte and glucose imbalance, and avoidance of sympathetic stimulation are all required for a parturient with thyrotoxicosis. Care should be provided in a critical care setting, with postpartum monitoring continuing. Pathological conditions resulting from iodine deficiency, hypothyroidism, or hyperthyroidism are important in anaesthesia and should be addressed with the physiological changes of pregnancy in mind. In patients with autonomic dysfunction due to diabetes mellitus or diabetic ketoacidosis, GA can disguise the signs and symptoms of hypoglycemia, while neuraxial anaesthesia can cause exacerbated haemodynamic instability [24].
7. CHANGES IN THE CENTRAL AND PERIPHERAL NERVOUS SYSTEMS
During pregnancy, the central and peripheral nerve systems experience considerable modifications. Cerebral blood flow is boosted as cerebrovascular resistance is reduced. The blood-brain barrier becomes more permeable. During pregnancy, the minimum alveolar concentration (MAC) drops by 25-40% [85].
Increased levels of plasma endorphin and progesterone cause an increase in pain threshold at full term and during labor. Endorphin concentrations do not increase until the commencement of active labor, according to a few studies [86], therefore, this cannot explain early MAC decline. When oophorectomized rabbits were given exogenous progesterone, MAC was lower than in control animals [87]. When compared to nonpregnant age-matched controls, parturients had a larger dermatomal distribution of sensory anesthesia following epidural anesthesia [88]. Dilatation of the epidural venous plexus develops due to compression of the IVC by the gravid uterus [24]. A reduction in epidural space volume generated by an engorged epidural venous plexus due to aortocaval compression explained the disparity. Epidural fat is increasing, but epidural free space and spinal cerebrospinal fluid (CSF) volume are decreasing [89]. During pregnancy, CSF pressure remains constant, but it rises during uterine contractions and bears down. For the preservation of haemodynamics, there is a greater reliance on the sympathetic nervous system. A second study found that this difference remains even in early pregnancy (8-12 weeks) when the tiny gravid uterus is unlikely to cause mechanical blockage [90], and that epidural venous engorgement later in pregnancy appears to reduce CSF volume rather than epidural extravascular volume. Pregnancy-induced compensatory respiratory alkalosis, decreased plasma and cerebrospinal fluid (CSF) protein levels during pregnancy, resulting in higher free local anesthetic, and pregnancy hormones were the variables identified. Based on animal research, the latter is the most plausible explanation.
7.1. Clinical Implications Related Anaesthesia
As pregnancy advances, the anatomy and sensitivity to pain and medication change. Parturients are also more vulnerable to medicines that affect the central nervous system, with a 30% reduction in the MAC of inhalation anaesthetics [91]. Intravenous induction and sedative drugs are physiologically more sensitive in pregnant women [92]. Increased venous pressure below the gravid uterus causes blood to flow down the path of resistance, diverting it into the engorged epidural plexus.
When the epidural space is occupied, the volume of cerebrospinal fluid decreases to compensate. When compared to the nonpregnant state, this, combined with increased neural susceptibility to local anaesthetics and a higher apical level of thoracic kyphosis, results in a 25% reduction in the dose requirement for spinal and epidural anaesthesia, with a faster onset and longer duration of action. Since the conclusion of the first trimester, the spinal dose of local anaesthetic (LA) has decreased by 25-40%, indicating that changes in epidural space anatomy are not the main cause. Progesterone has been discovered to improve the susceptibility of neuronal membranes to LA [93]. Even though the specific mechanism of increased sensitivity of the central nervous system and peripheral nervous system to general and local anesthetics is unknown, it is generally recommended to reduce anesthetic doses in pregnant women, at least at first. It is unknown when these modifications revert to their nonpregnant condition due to a lack of evidence and confusion about the actual processes behind the heightened local anesthetic sensitivity during pregnancy. The sensitivity to spinal anesthetics appears to be normal 24-48 hours after delivery. Following sympathetic blockade caused by neuraxial anaesthesia, pregnant women are more susceptible to hypotension and haemodynamic instability. In isolated nerve fibers, enhanced sensitivity to bupivacaine has also been reported [94]. The enhanced susceptibility of the peripheral nervous system to anesthetics reported in parturients could be due to progesterone or one of its active metabolites. This enhanced sensitivity was also seen in nerves from oophorectomized rabbits who had been given exogenous progesterone for a long time [93]. Surprisingly, this behavior was not observed after acute progesterone intake [95]. Peripheral nerve sensitivity to local anesthetics has also been demonstrated in humans [96].
8. THE ANAESTHETIC IMPACT AND CLINICAL IMPLICATIONS
The anesthetic components of surgery take on major proportions depending on the procedure. Any elective general surgical operation should be avoided if at all possible during pregnancy. Emergency surgery can be cardiac or noncardiac, obstetric or non-obstetric, and has a variety of consequences. During the three trimesters, the anesthetic effect and clinical implications are slightly variable.
8.1. THE FIRST TRIMESTER
Physiological factors are significantly altered during pregnancy, altering normal reactions to anesthetic effects. The physiological changes in numerous organs during pregnancy are an adaptive response to the altered metabolic profile and the many demands and stresses that come with pregnancy. During the first trimester, the main goal is to prevent any substance or method that may interfere with appropriate embryological development. Because physiological functions are significantly altered after 6-8 weeks, oxygenation, normovolaemia, and stable haemodynamic parameters are the primary goals of anaesthetic administration at this time [4]. As an adaptation response, these physiologic alterations influence nearly all major organ systems, including the cardio-vascular, pulmonary, renal, hepatic, and neurological systems, and result in multiple metabolic changes [4]. Increased oxygen consumption is required to meet the increased demands of both maternal and foetal tissues, while a parturient's functional residual capacity is diminishing, making more prone to hypoxemia. Normal pregnant hyperventilation causes CO2 to be washed away, resulting in relative hypocarbia. Reduced minimum alveolar concentration, faster inhalational induction due to decreased functional residual capacity, rapid induction with soluble agents, and increased risk of hypoxemia due to decreased functional residual capacity and increased oxygen consumption are some of the notable and significant anaesthetic implications during this time [62]. Excessive mechanical breathing during GA might result in a drop in CO2, which causes severe vasoconstriction, lowering maternal cardiac output and jeopardizing uterine blood flow.
Titration of anaesthetic medications is essential because the requirements for both inhalational and intravenous drugs are reduced throughout this time. Increased blood plasma and RBC volume; increased cardiac output; increased heart rate; and benign arrhythmias such as ST, T, and Q-wave alterations, with left axis deviation in some parturients, are among the noteworthy cardiovascular abnormalities [97, 98]. The purpose of anaesthetic management is to distinguish these alterations from those caused by overt cardiac diseases such as systolic murmur (>grade III), diastolic murmur, and severe symptomatic arrhythmias.
Hormonal changes during pregnancy, in addition to the aforementioned alterations, might result in greater weight gain and vascularity. Breast enlargement, laryngeal oedema, and increased chest antero-posterior diameter damage the soft tissues of the neck, making airway management difficult. The oro-nasal airway must be meticulously instrumented due to the increased vascularity of the mucous membranes, which poses a greater risk of bleeding. Because plasma cholinesterase levels are reduced in more than 30% of parturients, patients given GA and succinylcholine may need to be ventilated for longer periods of time. Patients with a significant drop in plasma cholinesterase levels benefit greatly from neuromuscular monitoring [62, 99-103]. During this time, foetal monitoring should preferably be done using a Doppler ultrasound. Any type of emergency surgical surgery requires pre-, intra-, and post-operative foetal monitoring. Because of the problems generally associated with GA in this segment of the population, including difficult airways and a high risk of aspiration, regional anaesthetics should be the primary choice whenever possible [17]. During the first trimester, the danger of teratogenicity in the foetus is the highest, and all essential medicines should be delivered after a close discussion between the anaesthesiologist and the obstetrician.
8.2. SECOND TRIMESTER
To lessen the risk of spontaneous abortion and premature labor, it has been standard practice to postpone surgery until the second trimester. By this time, the majority of physiological changes have reached a plateau, making anaesthetic management safer than in the first or third trimesters. Although the second trimester is believed to be a safer phase for emergency surgery than the first and third trimesters, it is nevertheless connected with several substantial physiologic changes that can endanger both the mother's and the foetus' lives.
Airway management becomes even more difficult. The gravid uterus begins to compress the larger vessels, and aorto-caval compression can be quite dangerous when general or regional anaesthesia is administered. Acute appendicitis, cholecystitis, and intestinal blockage are the most common non-obstetrical surgical emergencies that complicate pregnancy. Torsion of ovarian cysts or masses, cholelithiasis, adrenal tumor, hernias, complications of inflammatory bowel disease, or stomach pain of uncertain aetiology are among the less common surgical diseases [3, 104].
A left lateral position or keeping a wedge under the right side while supine can help prevent aorto-caval compression. Due to the diminished capacity of the epidural space, venocaval compression causes distension of the epidural venous plexus, which raises the risk of inadvertent intravascular injection of local anaesthetics (LAs) as well as rapid distribution of the LA medication. During pregnancy, there is a significant increase in the levels of pro-coagulant factors, resulting in a hyper-coagulable state that begins in the late first trimester and reaches a plateau in the second trimester. These alterations necessitate thromboprophylaxis in high-risk patients who are more likely to have this problem after the second trimester [105].
8.3. THIRD TRIMESTER
In the third trimester, decision-making gets a little easier because a caesarean section can be performed before the major surgery. Due to increased airway oedema caused by hormone interactions, all additional challenges to airway control during the first two trimesters are amplified. An emergency operation after 28 weeks can be performed with the introduction of steroids, which give critical cover for foetal lung maturation and prevent the neonate from developing acute respiratory distress syndrome [99].
The precautions taken in the second trimester apply equally effectively in the third trimester, with a higher risk of aorto-caval compression due to a considerably larger gravid uterus than in the second trimester. Even during this time, regional anaesthetic is the recommended option due to the inherent danger of GA in parturients. GA should be used with caution because inhalational anaesthetics can cause significant uterine relaxation, which can lead to post-partum bleeding [106, 107]. For caesarean birth, analgesics, sedatives, and anaesthetics must be used with prudence because they can have a significant impact on infant health and Apgar scores. Several medicines, as well as surgical and anaesthetic stress, have the potential to suppress lactation, which may or may not be temporary, necessitating particular caution during anaesthesia. One major source of concern during this time is the likely emission of various medicines through breast milk, which might have negative effects on the newborn during nursing, particularly opioids and sedatives.
9.1. LABOUR ANALGESIA
Since its origin, both in industrialized and developing countries, labor analgesia has been connected with misconceptions and disputes. Since the early 1950s, when labor analgesia was first introduced into clinical practice, obstetrical anaesthetic science has changed dramatically because of the development of sophisticated procedures, monitoring devices, and the availability of newer, safer, and more effective medicines [108]. As a result, over the last two decades, the quality of labor analgesia methodology has vastly increased. The development of obstetrical anaesthesia as a subspecialty has aided the growth and advancement of labor analgesia science [109]. Although non-pharmacological treatments like water baths, intradermal sterile water injection, TENS, hypnosis, and acupuncture are still used in some parts of the world, pharmacological methods have been the cornerstone in the provision and advancement of labor analgesia [110].
Pethidine, fentanyl, tramadol, butorphanol, and remifentanil are examples of parenteral opioids that have been successfully utilized for labor analgesia, but no one agent is without adverse effects when given in greater dosages [111-114]. Inhalational medicines such as Entonox and the short-acting fluorine anaesthetic sevoflurane are less effective, but they are not without adverse effects, especially at higher doses [115, 116]. The breakthroughs in regional anaesthetic technology have taken labor analgesia to new heights. The use of lower doses of LAs and opioids in epidural and combination spinal epidural analgesia has proved revolutionary in reaching the intended goals of labor analgesia [117]. The modern methods of the drug delivery system, such as continuous epidural infusion using infusion pumps, patient-controlled analgesia, and computer-integrated, patient-controlled epidural analgesia, have mostly been essential in popularizing the science of painless labor [118-120]. The introduction of newer LAs such as levo, bupivacaine and ropivacaine has improved the safety of labor analgesia even further [121]. Adjuvants such as opioids, α-2 agonists such as clonidine and dexmedetomidine, neostigmine, and others enable not only the necessary analgesia to be achieved with lower dosages of LA but also the analgesic effect to be prolonged [122-125]. Overall, lower LA dosages have almost eliminated the different side effects associated with greater concentrations of the anesthetic drugs, making labor virtually painless. Although some studies indicate that pain-relieving interventions during labor are linked to a higher rate of operational delivery, there is no considerable literature to back up these claims [126].
9.2. ANAESTHESIA FOR OPERATIVE DELIVERY
A recent trend has shown an increase in the frequency of caesarean sections performed for one reason or another in both developed and developing countries [127-129]. Advanced and timely foetal monitoring, which diagnoses early foetal compromise and increases patient preference for operative delivery, are two more possible causes for these surgical treatments [106]. Now that it has been proven that regional anaesthesia is far safer than general anaesthesia for caesarean sections, the majority of operational operations for delivery are performed under regional anaesthesia around the world [130]. The type of anesthetic used is heavily influenced by maternal preferences, concomitant conditions, and the urgency of the surgery [131-133].
Whatever the form of anesthetic is used, the most important element is the reduced frequency of maternal deaths as a result of enhanced anaesthetic care, especially in developed countries [134]. GA is used in a variety of situations [135], including
- Patient's refusal of regional anaesthesia
- Coagulation abnormalities
- Various contraindications to regional anaesthesia, such as severe active infection in the back, neurological diseases, spinal deformities, and so on
- Foetal compromise requiring immediate operative intervention
The most difficult component of providing GA to an obstetric patient is managing a difficult airway [136]. The anatomic and physiologic changes that occur during pregnancy, such as soft tissue oedema of the upper airway, weight gain, breast enlargement, increased mucosal vascularity with an increased propensity to bleed, and a high risk of aspiration pneumonitis, make airway management extremely difficult [100]. Because the hormonal imbalance lowers the tone of the upper esophageal sphincter, even with sufficient fasting, there is always a danger of aspiration in these patients. Due to the prolonged gastric emptying time and intra-abdominal pressure caused by a gravid uterus, the risk of pulmonary aspiration is increased even more [137]. In such patients, quick sequence induction and intubation is the preferred approach to securing the airway, and a variety of anaesthetic methods are available to reduce the stress reaction associated with airway manipulation and intubation [138]. Newer supraglottic devices, such as the proseal laryngeal mask airway and the intubating laryngeal mask airway, have made administering GA and managing a difficult airway even easier [136, 139].
Not only is regional anaesthesia connected with the avoidance of airway manipulation, but it also has the advantage of avoiding the polypharmacy used in general anaesthetics. Regional anaesthetic also allows the parturient to stay awake during the surgical procedure and hear the baby's first cry, which can provide a psychological boost to nervous patients [106, 140]. Although both spinal and epidural techniques have proven to be effective, spinal anaesthesia is more common and important when a quick delivery is required, and the cost-effectiveness of spinal anaesthesia is more comforting to the patient's relatives than epidural, particularly in developing countries [130, 141]. The epidural is a more versatile approach because it can be utilized for labor analgesia and operational interventions using the same catheter if necessary. Many parturients prefer this procedure since it allows them to have a longer period of pain-free recovery. If the sensory level exhibits early regression during the surgical operation, this approach has the added benefit of extending the block height. Increased costs and a longer time to achieve a sufficient block are only a few of its major drawbacks [142]. Sensory anaesthesia is established in a significantly shorter time with the inclusion of adjuvants such as opioids and α-2 agonists and with a lower dose of Lasix (furosemide) [125]. The benefits of both spinal and epidural anaesthesia are combined in the combination of spinal epidural anaesthesia. It not only creates a quick and dense block but also a pain-free postoperative time with top-up dosages. The applicability of this methodology is particularly important in high-risk patients, such as those with heart disease, diabetes, or pre-eclampsia [143]. Although many anaesthesiologists favor GA for eclampsia patients, in problematic cases, a graded epidural approach with titration of the local anaesthetic dose is equally effective. Given the disordered pathophysiology in pre-eclampsia patients, achieving stable haemodynamics is critical [144].
9.3. ANAESTHESIA FOR POST-PARTUM PERIOD
The postpartum period begins after the placenta is delivered and can last up to 6 weeks. Surgical procedures should be avoided as much as possible during the first six weeks after delivery. During these 6 weeks, normal physiological parameters are being restored to prepregnancy levels, and as a result, anaesthetic responses can be unpredictable or exaggerated. The most prevalent emergency in the immediate postpartum period is a high risk of postpartum haemorrhage, which necessitates the use of an anaesthesiologist during the surgical intervention [145]. Furthermore, many tubal ligation procedures are performed around this time in underdeveloped countries like India to guarantee maximal patient cooperation for family planning aims. In the background of these alterations, the physiological changes match the early portion of the first trimester and have anaesthetic implications that are practically identical. Because only the mother body has to bear the burden of any procedure, the risk is often lessened. There is still a potential that medicines supplied as part of the therapy regimen will get into breast milk. Anatomical changes are noticeable after childbirth, which greatly aids tubal ligation. The abrupt rise in blood volume owing to auto-transfusion after birth, which can cause congestive heart failure, is the most important factor in patients with cardiac illness during this time. Because rapid infusion can cause a substantial lowering of systemic vascular resistance and increase of pulmonary vascular resistance, which leads to decreased cardiac output, the rate of oxytocin infusion must be controlled and should be given at 60-80 mIU/min. Methylergometrine and PGF2-α can produce significant hypertension, tachycardia, and increased pulmonary vascular resistance in cardiac patients, which can be highly dangerous.
10. DESCRIPTIONS ON ECLAMPSIA AND MANAGEMENT OF ANESTHESIA IN WOMEN WITH ECLAMPSIA
Any pregnant woman presenting with convulsion in an emergency setting should be taken as eclampsia unless proved. The Greek meaning of eclampsia is the fancied perception of flashes of light, as the entity is associated with visual disturbances. Eclampsia is defined as the occurrence of one or more generalized convulsions and/or coma in the setting of preeclampsia and in the absence of other neurologic conditions before, during, or after labor [146]. The differential diagnosis includes epilepsy, cerebral infarction, cerebral hemorrhage, subarachnoid hemorrhage, cerebral venous thrombosis, cerebral edema, malignant hypertension, benign and malignant cerebral tumors, cerebral abscess, viral, bacterial, parasitic infestations, hyponatremia, hypocalcemia, hypoglycemia, and hyperglycemia [147, 148]. Risk factors for eclampsia include nulliparity, multiple gestation, molar pregnancy, triploidy, preexisting hypertension or renal disease, previous severe preeclampsia or eclampsia, nonimmune hydrops fetalis, and systemic lupus erythematosus [149]. The hypothesis of the mechanism of endothelial damage leads to pre-eclampsia and eclampsia [150]. The characteristics of seizures specific to eclampsia are described as follows. It has an abrupt onset of facial congestion with eye protrusion, foam of the mouth, and biting of the tongue. It typically begins as facial twitching and is followed by a tonic phase that persists for 15-20 s. Then it progresses to a generalized clonic phase characterized by apnea, which lasts for approximately 1 min. The breathing typically resumes with a long stertorous inspiration and the patient enters a postictal state, with a variable period of coma. Cardiorespiratory arrest and pulmonary aspiration of gastric contents may complicate a seizure [151]. The major complications of eclampsia include HELLP syndrome, intrauterine growth retardation, abruption placentae, neurologic deficits, aspiration pneumonitis, DIC, pulmonary edema, renal failure and cardiac arrest [152]. Imaging is not necessary, as neurological abnormalities are transient in most cases. Moodley et al. [153], in their study on electroencephalogram and computerized cerebral tomography findings in eclampsia emphasized that imaging has limited clinical value and it can be performed on affected women with focal neurologic signs, atypical seizures, and/or delayed recovery. The role of anesthesiologists in eclampsia is to help the obstetrician to control and prevent further convulsions, control blood pressure, establish a clear airway, prevent major complications, to provide labor analgesia and to provide anesthesia for cesarean section.
10.1. CONTROL AND PREVENTION OF CONVULSIONS
The elementary concepts of seizure control are to prevent maternal injury, ensure oxygenation, provide cardiorespiratory support, and prevent aspiration. Magnesium sulfate (MgSO4) is the anticonvulsant drug of choice [151]. In the IV regimen (Zuspan), MgSO4 was given as a 4 g IV bolus followed by 2 g/h as an infusion. In the IM regimen (Pritchard), 4 g of 20% MgSO4 IV and 10 g of 50% MgSO4 IM are followed by 5 g IM every 4 h. Continuous infusion maintains a steady-state plasma concentration than the IM regimen. MgSO4 is continued for 24 h after the last fit or delivery, whichever is later. Side effects of MgSO4 therapy are potentiation of neuromuscular blockade, respiratory depression, hypotension, cardiac arrest, atonic PPH, and reduced beat-to--to-beat variability in the fetal heart rate. Hence, it is essential to monitor knee jerk, respiratory rate, and urine output during MgSO4 therapy. Serum Mg levels should be monitored in the IV regimen as the therapeutic window is very narrow. Therapeutic plasma level of Mg were 4-7 meq/l or 4.8-8.4 mg/dl (1 meq/l = 1.22 mg/dl). If the seizure continues, or if seizures recur, we give a second bolus (2 g) of magnesium sulfate. If seizures continue despite a further bolus of magnesium sulfate, treated with phenytoin (15 mg/kg) or diazepam (10 mg) or thiopentone (50 mg IV). The multinational Eclampsia Trial Collaborative Group study revealed that MgSO4 is superior to diazepam or phenytoin [154]. Resistant seizures should be managed by muscle relaxation and IPPV. The most common dilemma faced by critical care physicians is whether MgSO4 can be given in cases of reduced GFR. The initial 4 g loading dose of magnesium sulfate can be safely administered regardless the renal function. This is because after distribution, the loading dose achieves the desired therapeutic level and the infusion maintains the steady-state level. Thus, only the maintenance infusion rate should be altered with the diminished glomerular filtration rate. The renal function was estimated by measuring plasma creatinine. Whenever plasma creatinine levels are more than 1.0 mg/ml, serum magnesium levels are used to adjust the infusion rate.
10.2. CONTROL OF HYPERTENSION
The NICE Guidelines for hypertension management are [155]:
- Antihypertensive treatment is started when systolic blood pressure is over 160 mmHg or diastolic blood pressure is over 110 mmHg
- Consider treatment at lower levels if other markers of potentially severe disease like heavy proteinuria or disordered liver or hematological test results are present
- Labetalol, hydralazine, and nifedipine are commonly used drugs
- Atenolol, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor-blocking drugs (ARB), and diuretics should be avoided
- Nifedipine is to be given orally. There is no role for sublingual nifedipine
- Labetalol should be avoided in women with known bronchial asthma.
In an emergency setting, oral nifedipine 10-20 mg every 30 min with a max of 50 mg or inj. Labetalol 20, 40, 80, and 80 with a gap of 20 min intravenously based on the response to a maximum dose of 220 mg were given. Inj Hydralazine 5-10 mg every 20 min with a max dose of 20 mg can also be given. If the result is not satisfactory, the last option is intravenous nitroglycerine [151].
10.3. PREANESTHETIC EVALUATION
(1) The following is a brief list of possible problems when anaesthetizing a case of eclampsia [151]:
- Poorly controlled hypertension
- Albuminuria (decreased colloid osmotic pressure)
- Thrombocytopenia
- Central vascular depletion
- Associated systemic illnesses like diabetes mellitus
- Hypertensive response during intubation and extubation
- Drug interaction with magnesium sulfate
- Airway edema
- Thromboembolism.
(2) Assessment of target organ involved [151]:
- Cardiovascular system: Hypertension control, LV function, and intravascular depletion (check osmolality)
- Respiratory system: For signs of pulmonary edema
- Renal: Degree of oliguria and creatinine level
- Liver: Liver function test, clinical features of stretching of the liver capsule
- Coagulation profile: Platelet count, prothrombin time, activated partial thromboplastin Time
- Airway examination: Degree of airway edema
- ABG analysis: Acidemia.
(3) Goals of anesthesia management [151]:
- Seizure control
- Blood pressure control: Appropriate treatment must be instituted if the diastolic pressure exceeds 110 mmHg
- The possibility of increased ICP need not concern the anesthesiologist if the patient remains conscious, alert, and seizure free
- Persistent coma and localizing signs may indicate major intracranial pathology, which would affect anesthetic management
- Maintenance of fluid balance: intake should be restricted to 80 ml/h
- Monitoring of maternal oxygenation by continuous pulse oximetry
- Keep blood products available
- Coagulation studies should be undertaken regardless of the platelet count
- Judicious preloading of hypovolemia is suspected or post vasodilator therapy.
10.4. MANAGEMENT OF LABOR PAIN
Epidural analgesia is possible in conscious eclamptic women with no evidence of increased ICP or coagulopathy and whose seizures have been well controlled. Most of us avoid epidural if a neurologic deficit exists until the diagnosis becomes clear. The technique is as follows [151]. To start, hydration with 0.5-1 L of crystalloid is necessary. Maternal electrocardiogram, blood pressure, as well as fetal heart rate, should be monitored continuously. Administration of oxygen with a facemask or nasal cannula is beneficial. Among the local anesthetics, a low concentration of bupivacaine, 0.125%, with 2 μg/ml of fentanyl as an initial bolus, provides excellent analgesia with minimal motor block. Lesser the motor block, the greater the benefits regarding fetal head rotation. The mixture can also be given as epidural infusion at the rate of 10-12 ml/h. Other local anesthetics, such as ropivacaine and levobupivacaine may be used but the superiority of these agents compared to bupivacaine is not established until now. In the combined spinal epidural technique, the intrathecal dose, an opioid alone such as fentanyl or sufentanil may be used or a combination of 1.25-2.5 mg of bupivacaine with 25 μg fentanyl. Opioids, especially in large doses, are cautiously administered for the possibility of exacerbation of increased ICP from respiratory depression. It is better to avoid overzealous preloading with intravenous fluids before establishing low-dose epidural analgesia and combined spinal epidural analgesia. Newsome et al. [156], in their study on hemodynamic effects of lumbar epidural anesthesia in severe preeclampsia, have suggested that with judicious hydration and slow induction of block, hypotension can be minimized with little change in CVP, CI, and PCWP. Ergometrine should be avoided in the third stage of labor as it may elevate the blood pressure further. Instead, oxytocin 20 IU in a liter of Ringer’s lactate solution is to be given intravenously at 10 drops/min. The second stage is assisted by forceps in all eclamptic patients having a vaginal delivery to minimize maternal efforts at bearing down and prevent further increase in blood pressure [151].
10.5. MANAGEMENT OF ANESTHESIA FOR CAESARIAN SECTION
10.5.1. Regional Anesthesia
Spinal or epidural anesthesia can be given safely if the patient is conscious, seizure free, with stable vital signs, and with no signs of raised ICP. Moodley et al. [157] found no difference in maternal and neonatal outcomes when comparing epidural versus general anesthesia for cesarean section in conscious women with eclampsia. Spinal anesthesia with low-dose bupivacaine with fentanyl is a good option. Safety of spinal anesthesia has been studied in eclamptic by Razzaque et al. [158], who concluded that spinal is safer than GA for LSCS in eclamptic. A prospective cohort comparison by Antonie et al. [159] in patients with severe pre-eclampsia concluded that pre-eclamptic patients experience less hypotension during spinal anesthesia for elective cesarean delivery than healthy parturients. Hyperbaric bupivacaine (7.5 mg) with 25 μg fentanyl provides adequate anesthesia for cesarean section. If a CSE technique is instituted, the presence of the epidural catheter provides the flexibility to extend the level and the duration of the block. Contraindications to regional anesthesia include patient refusal, DIC, and placental abruption. Regarding administering spinal anesthesia in patients on aspirin, it has been recommended by the American Society of Regional Anesthesia that low dose aspirin therapy is not a contraindication to regional technique [54]. Regional anesthesia is considered safe when the platelet count is more than 75 000 per micro liter. A platelet count of more than 50,000 per micro liter is generally considered a contraindication. Within the range of 50-75 thousand per micro liter, an individual assessment (considering patient risk and coagulation tests) is necessary. The anesthesiologist should also maintain vigilance on the pulmonary function, urinary output, evidence of aortocaval compression, and epidural-induced systemic hypotension that may lead to decreased uteroplacental blood flow. Small incremental intravenous doses (50 ug) of phenylephrine may be used to treat hypotension temporarily while additional intravenous fluid is infused judiciously.
10.5.2. General Anesthesia
General anesthesia (GA) is the choice in the unconscious, obtunded patients with evidence of increased ICP. Anesthesia is achieved with an opioid and relaxant technique and deliberate hyperventilation [151]. The important considerations are
- Airway edema
- Possibility of difficult airway management
- Although cholinesterase levels decrease, the duration of action of succinylcholine and ester local anesthetics is seldom affected
- Exaggerated hypertensive responses to endotracheal intubation
- Drug interaction between magnesium and muscle relaxants
- A small dose of a volatile halogenated agent may prevent awareness
- Extubation is done in the left lateral position when the patient is fully conscious or else the patient is transferred to ICU for ventilatory support, depending on the preoperative condition and intraoperative behavior.
Initial finding seen in most cases is low CVP and high left-sided filling pressure (PCWP). If urine output is adequate, there is no necessity for any special monitoring. If urine output is not adequate, a fluid challenge is done with 250-500 ml of crystalloid infused over 20 min. If a response is seen, additional fluid boluses may be given cautiously. If there is no response to the initial fluid, bolus, CVP or PCWP, monitoring becomes necessary. Pulmonary artery catheter is indicated in severe pulmonary edema, oliguria unresponsive to fluid therapy, and intractable hypertension [160].
Currently, a volume expansion to CVP of at least 6-8 mmHg is considered safe and effective. Young et al. [161], in their study on hemodynamic, invasive, and echocardiographic monitoring in hypertensive parturients, found that the CVP-PCWP gradients in severe preeclampsia may be as high as 8-10 mmHg. Therefore, a CVP of 8 mmHg might correspond to a PCWP as high as 18 mmHg. This results in volume overload and possibly pulmonary edema. Hence, the aim of volume expansion to achieve a CVP of 4 mmHg or less may be better in eclamptic.
Even though individual cases differ, invasive blood pressure monitoring is required in the following situations [162].
- Sustained high BP
- Potential rapid BP fluctuations
- Inability to obtain BP by cuff
- Repeated sampling
- Use of peripheral vasodilators
- Concepts about pulmonary edema.
Pulmonary edema is a dangerous complication that may be either cardiogenic or noncardiogenic [163]. Cardiogenic pulmonary edema is due to either impaired left ventricular systolic or diastolic function. The presence of low CO, high PCWP, high CVP, and high SVR characterizes systolic dysfunction, whereas diastolic dysfunction is associated with normal or high CO, high PCWP, and normal SVR. Noncardiogenic pulmonary edema results from such factors as increased capillary permeability, iatrogenic fluid overload, an imbalance between colloid osmotic pressure (COP) and hydrostatic pressure, or a combination of these factors. The management varies according to the cause and clinical dysfunction.
In severe preeclampsia, vasospasm and endothelial dysfunction lead to a reduction in GFR. Rising serum creatinine and oliguria signal rapid deterioration of renal function. Clark et al., in their study on managing hemodynamic subsets of oliguria in preeclampsia, described three distinct types of hemodynamic findings [164]. The first group showed classic signs of hypovolemia as evidenced by low filling pressures, elevated SVR, and hyperdynamic cardiac function. These responded well to IV fluids. The second group showed normal or elevated filling pressures, elevated CO, and a high SVR. These patients were treated with vasodilators and fluid restriction. The third group showed elevated SVR and PCWP, and depressed cardiac function responded well to after-load reduction. So treatment for oliguria is situation based.
Women with pre-eclampsia are at increased risk of thromboembolic disease. Before delivery, all patients should have antiembolic stockings or low molecular weight heparin while immobile. Following delivery, low molecular weight heparin (dose adjusted on early pregnancy weight) should be given daily until the patient is fully mobile (seven days if delivered by Caesarean section). Low molecular weight heparin should not be given until 4-6 h after spinal anesthesia. An epidural catheter should be left in place for at least 12 h after low molecular weight heparin administration. Following removal of an epidural catheter, low molecular weight heparin should not be given for 4-6 h [54].
11. POSTPARTUM MANAGEMENT
In postpartum, close monitoring is done of vital signs, fluid intake and output, and symptoms for at least 48 h. These women usually receive large amounts of intravenous fluids during labor, delivery, and postpartum. In addition, during the postpartum period, there is the mobilization of extracellular fluid, leading to increased intravascular volume. As a result, women with eclampsia, particularly those with abnormal renal function, those with abruptio placentae, and those with pre-existing chronic hypertension, are at increased risk for pulmonary edema and exacerbation of severe hypertension [151]. Hence, it is essential to continue vigilance in the postpartum period. Regarding intravenous fluids, following delivery, the woman should be fluid restricted in order to wait for the natural diuresis that usually occurs sometime around 36-48 hours post-delivery. The total amount of fluid (the total of intravenous and oral fluids) should be restricted to 80 ml/h. Fluid restriction will usually be continued for the duration of magnesium sulfate treatment; however, increased fluid intake may be allowed at an earlier time point in the presence of significant diuresis. Parenteral magnesium sulfate should be continued for at least 24 h after delivery and/or for at least 24 h after the last convulsion [151]. Regarding antihypertensive therapy, methyldopa can be withheld in favor of calcium channel blockers, beta blockers, or alpha blockers.
CONCLUSION
To perform safe anaesthesia, you must have a thorough understanding of numerous physiological features of pregnancy as well as the pharmacological profiles of various medicines. One of the most significant changes during pregnancy is an increase in cardiac output. Normal pregnant women's femoral venous and inferior vena caval pressures rise significantly. Cardiac output rises to a maximum of 80 percent above pre-labor levels and around 150 percent above non-pregnant levels in the early postpartum period. I.V. and inhalation anesthetics, which cause a decrease in SV and CO, as well as neuraxial block, which causes sympathetic block, all enhance the risk of supine hypotension. Dilutional physiological anemia is caused by a disparity in the growth in plasma volume and red cell mass during pregnancy. Blood volume increases even more during labor. After delivery, the uterus involutes and placental circulation ceases, resulting in a blood autotransfusion of around 500 mL. The leukocyte count rises gradually and may rise even more during labor and delivery, but this is not a sign of developing sepsis. Most clotting factors and fibrinogen rise during pregnancy, but natural anticoagulants decrease and fibrinolytic activity decreases. When deciding on fluid and blood replacement in the peripartum phase, anesthesiologists should take into account the increased blood volume. At the end of the term, the functional residual capacity, expiratory reserve volume, and residual volume are all reduced. The vital capacity and the closure capacity remain unaffected. Because of the increase in chest circumference, total lung capacity is only slightly diminished. Reduced FRC shortens the time it takes to denitrogenate and accelerates the uptake of inhaled anesthetics. The nasopharyngeal approach should be avoided due to increased edema, vascularity, and friability of the mucous membrane. Nausea and vomiting affect the majority of pregnant women. The levels of serum albumin, transaminases, and bilirubin are all lower than they are in the non-pregnant condition. There's also evidence that gallstones are more common in pregnant women. Gastric emptying is delayed in the laboring patient, who may be experiencing cumulative effects from anxiety and labor pain. Preference for neuraxial procedures and the use of aspiration prophylaxis are important stages in prevention. In the absence of diabetes or renal illness, glucose and aminoaciduria can occur. During pregnancy, hydronephrosis and hydroureter are prevalent. Urinary incontinence, urinary tract infections, and nocturia are all common problems. By 4-6 weeks after delivery, all of the alterations in the renal system return to their pre-pregnancy status. Aldosterone's effects are amplified, resulting in increased water absorption, increased distribution volume, and increased elimination half-life of some medications. Thyroid stimulating hormone levels drop during the first trimester of pregnancy but then rise throughout the remainder of the pregnancy. Thyroid stimulating receptors (TSH) are stimulated by increasing levels of human chorionic gonadotropin, resulting in temporary hyperthyroidism and hyperemesis gravidarum. Due to the effects of placental lactogen, tolerance to a glucose load may be slightly decreased, resulting in a moderate diabetogenic state. To prevent premature labor, the oxytocin response to stress is reduced throughout pregnancy. An airway evaluation, ECG, invasive cardiovascular monitoring, treatment of hydration, electrolyte and glucose imbalances, and avoidance of sympathetic stimulation are all required for a parturient with thyrotoxicosis. During pregnancy, the central and peripheral nerve systems experience considerable modifications. Epidural fat is increasing, but epidural free space and spinal cerebrospinal fluid (CSF) volume are decreasing. For the preservation of haemodynamics, there is a greater reliance on the sympathetic nervous system. Intravenous induction and sedative drugs are physiologically more sensitive in pregnant women. Following sympathetic blockade caused by neuraxial anaesthesia, pregnant women are more susceptible to hypotension and haemodynamic instability. Because the physiological functions are severely disrupted, oxygenation, normovolemia, and stable haemodynamic parameters are the primary goals of anaesthetic administration at this time. Though the second trimester is considered a safer phase for emergency surgery than the first and third trimesters, it is still accompanied by some significant physiologic changes. Epidural and combination spinal epidural analgesia, which use lower doses of LAs and opioids to achieve the targeted labor analgesia goals, have proved revolutionary. Now that it has been proven that regional anaesthesia is far safer than general anaesthesia for caesarean sections, the majority of operational operations for delivery are performed under regional anaesthesia around the world. Surgical procedures should be avoided as much as possible during the first six weeks after delivery. The definitive treatment is delivery. However, it is inappropriate to deliver an unstable mother even if there is fetal distress. Once seizures are controlled, severe hypertension treated, and hypoxia corrected, delivery can be expedited. Always consider prophylaxis against thromboembolism. The fluid management along with magnesium and antihypertensive therapy with strict hemodynamic vigilance is to be continued in the postpartum period.
LIST OF ABBREVIATIONS
| AST | = Aspartate Aminotransferase |
| ALT | = Alanine Transaminase |
| ALP | = Alkaline Phosphatase |
| BUN | = Blood Urea Nitrogen |
| CO | = cardiac output |
| Cr | = Creatinine |
| CS | = Caesarean Section, also known as C-section |
| CSE | = Combined Spinal-Epidural technique |
| CSF | = Cerebrospinal Fluid |
| CVP | = Central Venous Pressure |
| DIC | = Disseminated Intravascular Coagulation |
| ECGs | = Electrocardiograms |
| EDV | = End-Diastolic Volume |
| GA | = General Anaesthesia |
| GFR | = Glomerular Filtration Rate |
| Hcg | = human Chorionic Gonadotropin |
| HELLP | = Hemolysis, Elevated Liver enzymes and Low Platelets |
| HR | = Heart Rate |
| ICP | = Increased Intracranial Pressure |
| ICU | = Intensive Care Units |
| i.v. | = Intravenous |
| LA | = Local Anaesthetics |
| LDH | = Lactate Dehydrogenase |
| LMWHs | = low Molecular Weight Heparins |
| LSCS | = Lower (uterine) Segment Caesarean Section |
| LOS | = Lower Oesophageal Sphincter |
| LV Function | = Left Ventricle Function |
| MAC | = Minimum Alveolar Concentration |
| MgSO4 | = Magnesium Sulfate |
| NMBAs | = Neuromuscular Blocking Agents |
| PaCO2 | = Partial Pressure of Carbon Dioxide |
| PCWP | = High left-sided Filling Pressures |
| RA | = Regional Anaesthesia |
| RBC | = Red Blood Cell |
| SV | = Stroke Volume |
| SVR | = Systemic Vascular Resistance |
| T3 | = Triiodothyronine |
| T4 | = Thyroxine |
| TEG | = Thromboelastography |
| TENS | = Transcutaneous Electrical Nerve Stimulation |
| TSH | = Thyroid Stimulating Receptors |
AUTHOR'S CONTRIBUTIONS
LM manuscript preparation and have read and approved the final manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
No funding was received for this study.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest regarding the publication of this paper.
ACKNOWLEDGEMENTS
The author is grateful to the College of Health Sciences Research and Community Office of Arsi University for their support and contribution.


