All published articles of this journal are available on ScienceDirect.
Consequences of Co-Administration of Propofol with Clonidine and Ketamine throughout Colon Cancer Surgery: A Randomized Trial
Abstract
Objective:
Analgesic effects can be further augmented with the addition of clonidine and ketamine to the TIVA propofol, providing an even more effective anesthetic without compromising patient safety. This study aims to determine whether propofol infusion combined with clonidine and ketamine is more efficient in lowering the level of IL-8, preserving operation stability, and dropping post-operative pain and morphine intake.
Methods:
We conducted a study in which two groups of 60 patients were scheduled for colorectal cancer surgery. The treated group, (group T), received premedication with clonidine, intraoperative ketamine, and propofol for sedation. As a control group, a normal saline solution was administered to the group (Group C).
Results:
Group T reported lower levels of post-operative pain than the control group (P<0.05). This suggests that group T was more effective at reducing pain than the control group. A significant difference in mean arterial blood pressure was observed between groups (P<0.05). It is worth noting that there was no statistically significant difference in IL-8 levels between the two groups postoperatively (P>0.05). There was also a lower consumption of morphine (4.09±1.78) in group T postoperatively.
Conclusion:
It was found that TIVA using propofol with clonidine and ketamine was more effective than propofol infusion alone in maintaining hemodynamic stability, reducing postoperative pain, and decreasing morphine consumption over conventional propofol infusion. As a combination, propofol, clonidine, and ketamine provide and manage the pain of patients in a synergistic manner.
Clinical Trial:
Registration no.: The trial was registered under the clinical trials registery NCTU5536362 at ClinicalTrials.gov.
1. INTRODUCTION
Surgery-induced trauma results in modifications to the immunological, endocrine, and metabolic systems [1]. Afferent neural stimulation from the surgical site, cytokine release from traumatised tissue, and activation of cellular and humoral immunological pathways all contribute to these changes [2]. Anesthesia, both general and regional, can reduce the stress reaction associated with surgical trauma. The anesthesia reduces the body's response to pain and can prevent the body from releasing stress hormones, such as cortisol and adrenaline, which can increase the body's stress response during surgery [3]. As a result of anesthesia's blocking of sympathetic and nerve fibers, the pituitary is altered, resulting in the adrenal gland's enlargement. Anesthesia today is dominated by total intravenous anesthesia, which involves administering propofol and opioids via target-controlled infusions. This is because total intravenous anesthesia is more reliable and predictable than other methods. It also allows for more precise control of the patient's level of anesthesia, as well as the ability to adjust the dosage as needed during the procedure [4].
The most widely used medication for total intravenous anesthesia (TIVA) is propofol. For TIVA, propofol is the most commonly used drug. Propofol has some advantages such as rapid onset and a quicker recovery process. With target control infusion (TCI) and TIVA, TCI plasma concentrations are expected to be more constant. In some studies, propofol TCI is combined with clonidine or ketamine to enhance its ability to cause the triad of anesthesia. Propofol is a short-acting drug, so it can be adjusted quickly as needed. Its rapid onset also means that it can achieve the desired level of anesthesia quickly and without much risk. Additionally, its shorter recovery time allows the patient to wake up more quickly, which is desirable in many cases. Furthermore, its ability to maintain more constant plasma concentrations makes it a good choice for TCI and TIVA. Finally, the combination of propofol TCI with other drugs such as clonidine or ketamine can be beneficial in some cases, as it can improve the efficacy of the overall anesthetic [5]. It is anticipated that using TIVA with target control infusion (TCI) will give the drug's plasma concentration more consistency. In certain trials, propofol is combined with medications that work synergistically, such as clonidine or ketamine, to increase the drug's capacity to produce the trio of anesthesia [6].
The use of clonidine as a premedication may lower a patient's dose of volatile or intravenous anesthetics since it has various effects that include sedation, analgesia, and sympatholysis. Clonidine is a sedative and analgesic drug that can help ease anxiety and pain in a patient before a surgical procedure. It also has the ability to reduce the amount of anesthetic needed for the procedure. This is because it has the ability to reduce the activity of the sympathetic nervous system, which helps decrease blood pressure, heart rate, and other vital signs [7].
Ketamine has the ability to inhibit NMDA receptor-mediated neurotransmission, which can lead to decreased sympathetic activity. This can result in decreased peripheral vascular resistance and increased cardiac output, leading to increased hemodynamic stability. The combination of ketamine and opioids can also produce an analgesic effect [8]. For instance, the combination of ketamine and morphine can produce a synergistic analgesic effect that is greater than the sum of the two drugs taken alone [9].
Therefore, the purpose of this study is to define whether combining propofol infusion with clonidine and ketamine is more efficient at lowering the level of Interleukins 8 (IL-8), preserving intraoperative hemodynamic stability, and minimizing postoperative discomfort and using morphine.
2. PATIENTS AND METHODS
The study was conducted in a double-blind, randomized controlled group. In this case, randomization was created using a computer-generated randomization list. Furthermore, the use of a double-blind procedure ensured that neither the participants nor the researchers were aware of the group assignments. All subjects' approval was granted by the October 6 University Hospital's ethical committee. The trial was conducted in accordance with the GCP guidelines and the principles of the Helsinki Declaration. The trial was registered under the clinical trials registry NCT05536362 at ClinicalTrial.gov. To be a part of this study, every participant has given their written consent.
For this study, sixty contestants with ASA physical status I and II, ranging in age from 35 to 65, were scheduled for colon cancer operation lasting longer than 120 minutes. This study excluded participants with a history of heart, renal, and liver cell failure, allergic reaction to studied drugs, and history of epilepsy, hemodynamic instability, chronic pain, or mental illness. Using a computer-generated randomization list, the patients were randomly allocated into two groups (30 patients each) (Fig. 1). The treated group (Group T), got intravenous anesthesia with TCI propofol mixed with clonidine and ketamine, and the control group (Group C), received intravenous anesthesia with TCI propofol mixed with a placebo (NaCl 0.9%) . A third party was included in this study so neither the researchers nor the research subjects are aware of the intervention which the patients got.
Patients in the treated group received premedication with clonidine 1mg/kg in NaCl 0.9% for 10 min after taking blood samples for preoperative IL-8 levels. Anesthesia was induced by Propofol TCI plasma mode target (Mindray, Egypt) of 1-2 ug /kg. Fentany l-2 ug /kg and atracurium 0.5mg/kg was administered prior to endotracheal intubation. Ketamine was administered continuously during surgery at a rate of 10 mg/kg/min by a syringe pump, and propofol TCI concentration was adjusted to keep a bispectral index (BIS) value of 50-60 [10]. A second blood test for IL-8 was performed 24 hrs following the operation. Utilizing morphine as a patient-controlled analgesia (PCA) to treat postoperative pain (demand dose of 1 mg, lockout interval at 4 minutes, and the maximum dose for 4 hrs is set at 10 mg).A visual analogue scale(VAS)was used to measure the amount of PCA used and the severity of the pain [11]. Enzyme-linked immunosorbent assay (ELISA) was performed to assess IL-8 levels [12]. Patients in the C group, received all treatments similar to that in the T group, except for the administration of pre-operative clonidine and intra-operative ketamine.
Regarding the calculation of the sample size, according to the total morphine consumption of the groups of interest, Epi Info STATCALC was used to calculate the sample size. We calculated a sample size of 60 patients undergoing colorectal cancer surgery (30 in each group) with a power of 90% (power of test) and a type I error of 0.05. Based on Epi-Info's output, 60 patients were taken as the sample size. By 1:1 ratio, we randomized 60 cases into two groups (30 cases in each group).
3. RESULTS
Subject characteristics are shown in Table 1. Age, gender, body mass index (BMI), and physical state ASA parameters are non-significant in both groups (P>0.05). In groups C and T, the average ages were 49.8 and 48.2 years, respectively. In group T, the mean BMI was 21.7 kg/m2, and in group C, it was 24.5 kg/m2. Collectively, these results suggest that these characteristics did not affect the outcome of the study.
| Parameters |
Group C (n=30) |
Group T (n=30) |
P-value |
| Age (years), mean ± SD | 49.8±1.53 | 48.2±2.26 | 0.87 |
| Gender, male, n (%) | 18 (60.0%) | 12 (40.0%) | 0.03 |
| BMI (kg/m2), mean ± SD | 24.5±2.09 | 21.7±1.12 | 0.65 |
|
ASA classification, n (%) I II |
15 (50%) 15 (50%) |
11 (36.7%) 16 (53.3%) |
0.06 0.16 |
At 6, 8, 12, and 24 hrs after surgery, postoperative pain was assessed using the VAS scale. Table 2 showed Mean±SD for the VAS data in our study. The results of the VAS scale indicated that there were significant differences between the two groups in terms of the level of postoperative pain experienced. The data showed that the group who had the surgery 6, 8, 12, and 24 hrs after surgery experienced a higher level of postoperative pain than the group who had the surgery within 6 hrs of surgery.
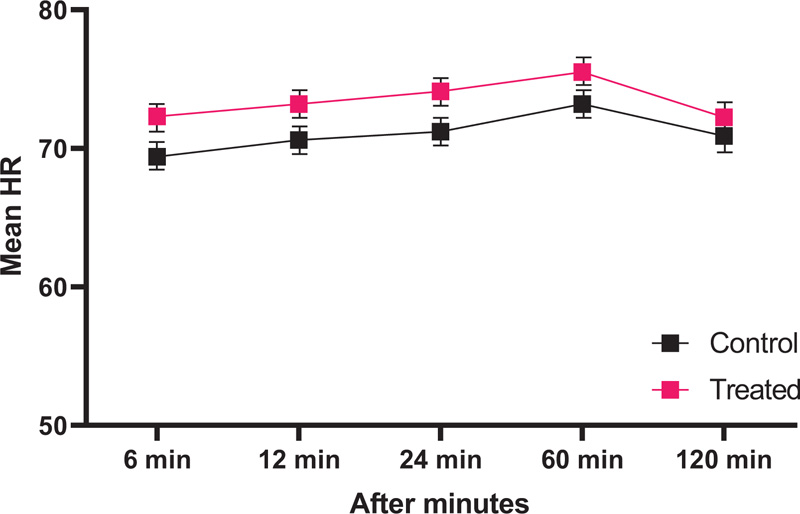
At 6 minutes, 12, 24, 60, and 120 minutes after the incision, both groups' mean arterial blood pressure and heart rate were monitored. All data are shown in Table 3 and Fig. (2). The data showed that there was a slight decrease in mean arterial blood pressure and heart rate for both groups as time passed. This suggests that the mean arterial blood pressure in group T was affected by the surgical incision, leading to a higher initial reaction, but eventually recovering more quickly than in group C (P<0.05).
| Outcome of VAS |
Group C (n=30) |
Group T (n=30) |
P-time | P- value (Time#) |
| VAS | - | - | - | - |
| - 6 hr | 4.88±0.81 | 3.02±1.01 | <0.001 | <0.001 |
| - 8 hr | 4.32±1.12 | 2.97±0.82 | <0.001 | <0.001 |
| - 12 hr | 3.22±0.11 | 2.14±0.16 | <0.001 | <0.001 |
| - 24 hr | 1.99±1.02 | 1.43±0.14 | <0.001 | 0.02 |
|
Group C (n=30) |
Group T (n=30) |
P-value (Time) | |
| Characteristics | - | - | - |
| 6 min | 110.2 ± 11.3 | 116.4 ± 11.4 | <0.01 |
| 12 min | 114.4 ± 13.7 | 119.3 ± 10.6 | <0.01 |
| 24 min | 121.2 ± 10.2 | 111.4 ± 12.1 | <0.01 |
| 60 min | 122.4 ± 9.30 | 120.4 ± 10.2 | <0.01 |
| 120 min | 116.5 ± 10.2 | 115.5 ± 8.9 | 0.04 |
| Characteristic |
Group C (n=30) |
Group T (n=30) |
95% CI | P- value |
| Preoperative IL-8 | 26.32±4.46 | 19.90±3.56 | (13.3-66.3) | 0.16 |
| Postoperative IL-8 | 34.41±1.59 | 33.24±1.56 | (11.7-99.6) | 0.47 |
| Morphine taken (mg) | 10.45± 2.31 | 4.09±1.78 | - | <0.001 |
The differences between the two groups did not reach statistical significance when the inflammatory cytokine marker IL-8 was assessed. Based on the total amount of morphine consumed, the total amount of morphine consumed in both groups was computed as significant as shown in Table 4. This suggests that the differences between the two groups in terms of the amount of morphine consumed were significant, even though the differences between the two groups in terms of the IL-8 marker were not. Furthermore, the data suggested that there is no correlation between IL-8 levels and post-operative morphine intake and that the difference in IL-8 levels between the two groups could be attributed to other factors such as differences in pre-operative health status.
4. DISCUSSION
Anesthesia affects cytokines based on medication and patient response. In comparison with volatile inhalational drugs, propofol may block and limit the proinflammatory cytokine IL-8 [13]. The production of IL-8 is claimed to be increased by ketamine. To reduce the sympathetic response of the central nervous system (CNS), clonidine, a selective partial alpha-2 agonist, was previously utilised as an antihypertensive medication [14]. Sedation, analgesia, antianxiety, lowering the anaesthetic dose, and perioperative hemodynamic stability are among the other known effects of clonidine [15]. The sympathomimetic effects of ketamine include elevated blood pressure, heart rate, cardiac output, and systemic vascular resistance [16].
There is a diametric difference between clonidine and ketamine. We can anticipate sedative, analgesic, and hemodynamic stabilising effects from mixing the two medications. In this study, at each predetermined time of measurement, we additionally compared changes in SBP and HR. Hemodynamic stability, one of the clonidine's effects, was attained in this study. Clonidine helps keep SBP and HR steady by reducing the intraoperative sympathetic response [17]. In this investigation, there were no considerable differences in IL-8 levels between the two groups (p > 0.05). There is evidence that propofol reduces IL-8 production, while clonidine lowers sympathetic activity in the central nervous system, it also lowers the neuroendocrine stress response that results from the autonomic nervous system being activated [18]. The effects of ketamine alone are often distinct from those of sympatholytic drugs like propofol and clonidine, which can also boost IL-8 production [19].
When clonidine is administered above the level of the spinal cord, it affects the brainstem's lateral reticular nucleus and the alpha-2 postsynaptic adrenoreceptor, which together reduce sympathetic tone. At the peripheral level, it has an impact on the presynaptic alpha-2 adrenoreceptor, lowering the release of norepinephrine in the sympathetic nerve terminals, resulting in blood vessel dilatation, and lessening the chronotropic influence on the heart [20]. Contrary to peripheral vasoconstriction caused by clonidine's direct activation of the receptors alpha-2 and alpha-1, these supraspinal and peripheral effects are caused by the drug [21].
The intravenous anesthetic medication ketamine can raise heart rate, blood pressure, cardiac output, cardiac contractility, and systemic vascular resistance. It has an indirect effect as a result of elevated central catecholamine from the adrenal medulla and elevated central sympathetic tone [22]. Adults who took ketamine at the clinical dose experienced an increase in SBP of 20-40 mmHg along with a small rise in diastolic blood pressure. Following an intravenous ketamine injection, systemic blood pressure typically increases gradually during the first three to five minutes before gradually declining for the following 10 to 90 minutes.
It is anticipated that the combined effects of clonidine and ketamine will produce a balance between effects that reduce sympathetic activity and effects that promote activity. Using PCA morphine at a PCA dose of 1 mg, lock-out intervals of 6 minutes, and a maximum dose of 10 mg/4 hrs, postoperative analgesia was administered to all individuals. At each measurement time, VAS scores were statistically different (p<0.05). Ketamine is given intravenously at a constant rate of 5-20 mcg/kg/min for sedation and analgesia, and intramuscularly at a rate of 2-4 mg/kg. Ketamine analgesia was accomplished at plasma values of 100-150 pg/ml. Ketamine at a dose of 50 g/kg did not sufficiently reduce pain, but it did cause drowsiness. After the initial bolus, a continuous infusion at a dosage of 3-4 g/kg/min or 14 g/kg/min can be used to achieve these plasma concentrations.
Because clonidine and ketamine work together [23] synergistically to reduce postoperative pain, the VAS score in group T receiving both medications was lower. The total amount of morphine consumed as recorded in the PCA machine is used to compute morphine consumption. When compared to the control group, group T's mean 24-hour morphine intake is reduced to 4.09±1.78 mg. These findings are in line with the postoperative VAS scores for the two groups.
Based on the limitations of this study, we found that the sample size was too small to detect any significant differences in the occurrence of side effects between the two groups. This means that the degree of pain scores could not be used as an indicator of an individual's risk of experiencing side effects. Additionally, the duration of the drug's effect on patients was not long enough to establish its systemic effect. This means that the drug's potential for long-term systemic effects remains unclear and requires further study. It is also possible that the results of the study were not generalizable because of the small sample size. Therefore, larger studies with more participants would be needed to draw more reliable conclusions.
CONCLUSION
While TIVA with propofol infusion and preoperative clonidine and intraoperative ketamine have been found to be advantageous in terms of hemodynamic stability, post-operative pain, and reducing morphine usage, the impact on the levels of pro-inflammatory cytokines, such as IL-8, has yet to be established. It is possible that the study was too short to measure any changes in pro-inflammatory cytokines or that the dose of propofol was too low to have an effect on these cytokines. It is also possible that the other drugs used in the TIVA protocol, such as clonidine and ketamine, may have had a direct effect on reducing the levels of pro-inflammatory cytokines.
LIST OF ABBREVIATIONS
| TCI | = Target Control Infusion |
| BIS | = Bispectral Index |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The October 6 University Hospital's ethics committee gave its approval, and the procedure was approved.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans were used in accordance withthe ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931) and GCP guidelines.
CONSENT FOR PUBLICATION
To be a part of this study, every participant has given their written consent.
STANDARDS OF REPORTING
CONSORT guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author [N.E].
FUNDING
None.
CONFLICT OF INTEREST
There are no disclosed conflicts of interest for the author.
ACKNOWLEDGEMENTS
Declared none.


