All published articles of this journal are available on ScienceDirect.
Early Use of Ibuprofen in Moderate Cases of COVID-19 Might be a Promising Agent to Attenuate the Severity of Disease: A Randomized Controlled Trial
Abstract
Introduction:
Critically ill COVID-19 patients undergoing cytokine storm are believed to have a worse prognosis and increased fatality rate. Ibuprofen is a non-steroidal anti-inflammatory drug (NSAIDs) that might prove beneficial for the early management of COVID-19 due to its immunomodulatory effects. This study aimed to assess the efficacy and safety of the early use of ibuprofen to attenuate the severity of the course of COVID-19 and improve outcomes in patients diagnosed with a moderate case of COVID-19 disease.
Methods:
This randomized, double-blinded prospective study was conducted from January, 2022 to May, 2022, which included a total sample size of 180 patients with moderate cases of COVID-19. The number of patients transferred to intensive care was used as a primary outcome with a proposed large effect size (0.8), alfa =0.05, and power=0.80, so 90 cases were included in each group. Secondary outcomes were inflammatory markers: C-Reactive Protein (CRP), serum ferritin, and interleukin-6 (IL-6), duration of hospital stay, and need for ICU admission.
Results:
One hundred eighty patients with moderate case of COVID-19 disease were divided in a 1: 1 ratio to receive ibuprofen (IG) or paracetamol (CG). The average age of the included patients was almost 41 years. Statistically significant differences were reported between both groups in terms of improvement in cough symptoms and lymphopenia in IG compared to CG (p= 0.034 and p= 0.044, respectively). Regarding secondary outcomes, statistically, significant differences were reported between the study’s groups in terms of the mean number of patients transferred to the ICU in IG compared to the CG (p =0.0.047) and duration of hospitalization (p =0.013), with no significant differences (p > 0.9999) in the occurrence of side effects.
Concerning the follow-up of the cytokine storm marker, there was a statistically significant reduction in serum cytokine marker compared to the baseline value (P < 0.05) in the same group. No statistically significant differences were observed when comparing both groups together in terms of serum ferritin level (p =0.570), serum IL-6 level (p =0.580), and CRP level (p =0.401).
Conclusion:
It can be concluded that early use of ibuprofen as adjuvant therapy in COVID-19 management is effective and safe to attenuate the severity of diseases and improve outcomes.
Trial Registration:
Project manager for the Pan African Clinical Trial Registry PACTR202202880140319. Registered 9th February, 2022 - Retrospectively registered, (https://pactr.samrc.ac.za/)
1. INTRODUCTION
Although established theories suggest an immune-inflammatory model of the pathogenesis of Coronavirus disease 2019 (COVID-19), a better understanding of the pathogenesis would eventually lead to an improvement in the treatment protocols, as well as the control of this pandemic [1].
Cytokine storm is an acute hyperinflammatory response that may be accountable for critical illness in many situations, including viral infections, sepsis, cancer, and multi-organ failure. The phenomenon has been linked to critically ill subjects infected with SARS-CoV-2, the novel coronavirus implicated in COVID-19. Critically ill COVID-19 patients suffering from cytokine storm are found to have a worse prognosis and increased mortality rate. In SARS-CoV-2 infected patients, cytokine storm appears important to the pathogenesis of several severe manifestations of COVID-19, including acute respiratory distress syndrome, thromboembolic events, such as acute ischemic strokes caused by large vessel occlusion and myocardial infarction, vasculitis, acute kidney injury, and encephalitis (Kawasaki-like syndrome in children and renal vasculitis in adult) [2].
Ibuprofen is a non-steroidal anti-inflammatory drug (NSAIDs) that might prove valuable for the early management of COVID-19 due to its immunomodulatory effects [3].
This study aimed to assess the efficacy and safety of the early use of ibuprofen to attenuate the severity of the course of COVID-19 and improve outcomes in patients diagnosed with a moderate case of COVID-19 disease.
2. METHODS
2.1. Ethical Considerations
This study obtained ethical approval from the Research Ethics Committee, Faculty of Medicine, Ain Shams University, Egypt. The trial was registered at the Pan African Clinical Trial Registry (http://pactr.samrc.ac.za) (PACTR202202880140319). Informed written consent was obtained from each participant. The confidentiality of the data was preserved. The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
2.2. Study Design
This randomized, double-blinded prospective study was conducted between January, 2022 to May, 2022 at Ain Shams University Hospitals' Intermediate Care Units on patients diagnosed with a moderate case of COVID-19 disease according to Egyptian national guidelines for COVID-19 [4]. A case was established to be moderate according to the following parameters (simply pneumonia without hypoxia):
1- Cough, fever (above 38°C), body aches.
2- Positive imaging in CT chest multifocal bilateral patchy ground-glass opacities (GGOs) or consolidation with interlobular septal and vascular thickening, mostly in the peripheral fields of the lungs (COVID-19 Reporting and Data System (CO-RADS-5-4)).
3- Spo2 ≥ 92%.
4- Confirmed PCR.
A total sample size of 180 discharged patients was used as a primary outcome with a proposed large effect size ((0.8), alfa = 0.05, and power = 0.80. Hence, 90 cases were included in each group. Secondary outcomes were inflammatory markers (serum ferritin, CRP, and Il-6), duration of hospital stay, number of patients transferred to ICU, and number of patients needing oxygen supply (high flow nasal cannula, non-rebreathing mask, non-invasive CPAP, or mechanical ventilation) to maintain oxygen saturation above 92%.
2.3. Sample Size Calculation
The sample size was calculated using NCSS PASS 11.0, as explained in a study carried out by Rinott et al., in 2020. The group sample size was 180 patients in total; 90 in group one (Ibuprofen group) and 90 in group two (Paracetamol group) to achieve 80% power to detect a difference between the group proportions of 0.1090. The proportion in group one (the treatment group) was assumed to be 0.200 under the null hypothesis and 0.1290 under the alternative hypothesis. The proportion in group two (the control group) was assumed to be 0.200. The sample size was inflated by 15% to account for the attrition problem in prospective studies.
2.4. Randomization and Allocation Concealment
We used the sealed, opaque, sequentially numbered envelopes method for randomization and allocation concealment of patients included in this trial. We used 180 identical, opaque envelopes lined with sheets of aluminum foil to ensure opacity. We prepared 180 envelope-sized sheets of white paper as well as 180 sheets of single-sided carbon paper, which were used to make 90 Treatment A and another 90 Treatment B envelopes. The two sets of envelopes were combined and shuffled thoroughly. Using a pen, we marked a number on the front of each envelope sequentially from 1 to 180, so the carbon paper inside the envelope transferred this number to the allocation paper inside. Finally, we placed all envelopes into a large plastic container, in numerical order, ready for use.
2.5. Eligibility Criteria
We enrolled adults of both genders, aged 18-60 years old, who had a moderate case of COVID-19.
We excluded patients with a severe case of COVID disease, advanced liver disease, renal dysfunction, history of peptic ulcer disease, and history of allergy to ibuprofen or paracetamol. Patients who received even one dose of any vaccine (SARS-CoV-2 vaccine) were excluded from this study. Any patient with any condition that impeded the provision of informed consent was excluded.
2.6. Interventions
Eligible patients were randomly allocated to receive either standard protocol for the treatment of COVID-19 patients using paracetamol (500 mg/6h) (Control group [CG], n = 90) or using ibuprofen (400 mg/6h) (ibuprofen group [IG], n = 90). Both groups medicated according to Egyptian protocol (Hydroxychloroquine 500 mg/12 hrs, Azithromycin 1gr first day then 500 mg per day for 3 days 1st line or Clarithromycin 500 mg every 12 hrs for 7-14 days, Oseltamivir 150 mg every 12 hrs for 5 days, Ascorbic acid 500 mg every 12 hrs, Cyanocobalamin IV once daily).
The entire clinical team and the data collectors and outcome adjudicators were not aware of which patient received which treatment assignment.
2.7. Study Procedures
On arrival to the intermediate care unit, patients were monitored with 5 leads ECG, pulse oximetry, and noninvasive blood pressure. Twelve leads ECG and chest X-ray were done, and a blood sample was taken for complete blood count, biochemistry, and arterial blood gas analysis.
Demographic data for all patients were collected. Additionally, the following data were recorded every 6 hours for clinical purposes during the first 24 hours and then again, every 24h for our study:
1- Body temperature.
2- Systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and heart rate (HR).
3- Respiratory rate (RR), SPO2.
4- Severity of cough (Severity of cough was defined as follows: 0--no cough at all; 1--occasional hems; 2--mild, isolated coughs, without additional symptoms; 3--moderate, paroxysmal coughs, without additional symptoms; 4--severe, strenuous coughs, accompanied by chest discomfort) [5].
5- Side Effects: stomach pain, nausea, vomiting, and renal impairment.
6- PO2/FIO2 ration every day.
7- Cytokine markers (serum ferritin, CRP, Il-6) on days 1 and 4.
2.8. Study Outcomes
2.8.1. A. Primary Outcome:
Number of patients transferred to ICU (if the condition becomes severe according to Egyptian National guidelines for COVID-19 (2020).
- Respiratory rate above 30 breaths per min, signs of severe respiratory distress (dyspnea, orthopnea), oxygen saturation (SPO2) (65-85).
- Complete blood picture, platelets fall to 70000 per ul, lymphopenia less than 6%, agranulocytosis (75-85).
- Raising CRP C-reactive protein.
- Deterioration of renal or liver function.
- ABG shows respiratory failure type 1 and respiratory alkalosis due to dyspnea and tachypnea.
2.9. Statistical Analysis
Results were expressed as mean ± standard deviation or number (%). Comparison between categorical data was performed using the Chi-square test. Comparison between different numerical data in the two studied groups was performed using an unpaired t-test. Comparison between different times of measurements and baseline within the same group was performed using repeated measure ANOVA. SPSS software package (SPSS for Windows®, Version 16.0. Chicago, SPSS Inc.) computer program was used for data analysis. A P-value less than 0.05 was considered significant.
3. RESULTS
This randomized, double-blinded prospective study was conducted from January, 2022 to May, 2022. A total of 233 COVID patients were assessed for eligibility, but only 180 patients were included in the final study (Fig. 1).
Table 1 shows equal distribution of sex, with comparable means of the age, weight of the patients, and associated comorbidity in both groups (p>0.05).
Considering the baseline clinical parameters, there were no statistically significant differences between both groups in terms of dyspnea (p =0.821), fever (p =0.858), cough (p =0.765), lymphopenia (p =0.828), positive CT finding (p =0.855), SPO2 (p =0.187), and PO2/FIO2 ratio (p =0.859) (Table 2).
Additionally, there were no statistically significant differences between both groups in terms of serum ferritin level (p =0.513), serum IL-6 level (p =0.436), and CRP level (p =0.452) (Table 3).
Regarding primary outcomes, there was a statistically significant reduction in the mean number of patients transferred to the ICU in the ibuprofen group compared to the control group (10 (11.1%) versus 21 (23.3%), p=0.047)). There was a significant improvement in cough symptoms (in terms of a decrease) and lymphopenia (an increase in lymphocyte count) in the ibuprofen group compared to the control group (p= 0.034 and p= 0.044, respectively). Duration of hospitalization was significantly different in the ibuprofen group (5.94 ± 4.582) versus the control group (8.06 ± 6.537) (p = 0.013) (Table 3).
Regarding secondary outcomes, a statistically significant reduction in serum cytokine marker was observed when compared to the baseline value (p < 0.05) in the same group. No statistically significant differences were observed when comparing both groups together in terms of serum ferritin level (p = 0.570), serum IL-6 level (p = 0.580), and CRP level (p = 0.401) (Table 4).
The frequencies of nausea and vomiting were equal in both groups (p>0.999). One patient in the ibuprofen group showed early renal impairment as in the control group (p=1.000) (Table 5).
4. DISCUSSION
In our study, ibuprofen was used in cases of COVID-19, a rapidly spreading global threat that has been announced as a pandemic by the WHO. COVID-19 is transmitted via aerosol transmission, resulting in pneumonia in most cases and acute respiratory distress syndrome (ARDS) in about 15% of the cases. Mortality in COVID-19 patients has been linked to the occurrence of the “cytokine storm” induced by the virus. Excessive production of pro-inflammatory cytokines leads to ARDS aggravation and widespread tissue damage, resulting in multi-organ failure and death. Targeting cytokines during the management of COVID-19 patients could enhance survival rates and lessen mortality [6].
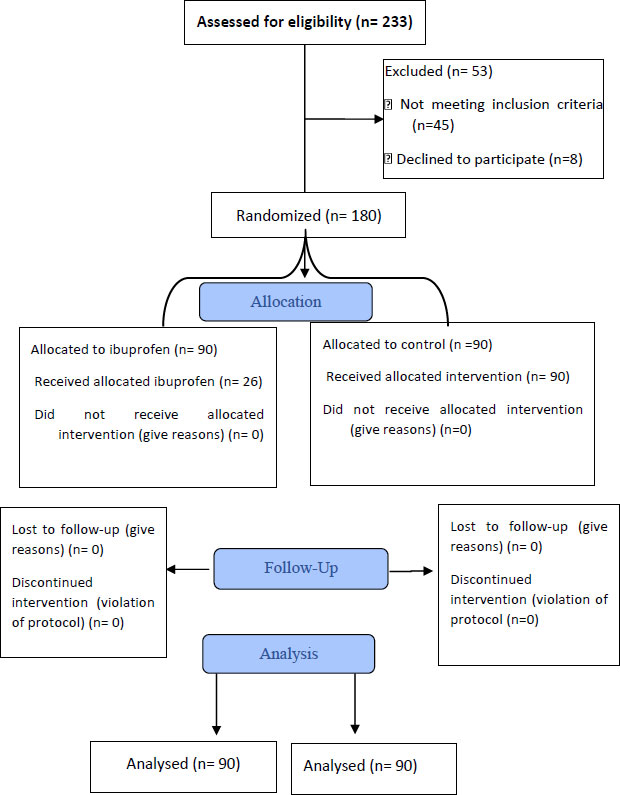
Table 1.
| Variables | Ibuprofen Group (N = 90) | Control Group (N = 90) | P-value | ||
|---|---|---|---|---|---|
| No | % | No | % | ||
|
Age in year - Mean (SD) |
41.71 (7.405) | 42.01 (6.468) | 0.773 | ||
|
Weight in Kg - Mean (SD) |
75.78 (11.220) | 75.59 (9.393) | 0.903 | ||
| Gender | |||||
| - Male | 43 | 47.7 | 41 | 45.5 | 0.881 |
| - Female | 47 | 52.3 | 49 | 54.5 | |
| Comorbidity | |||||
| - DM | 29 | 32.2 | 31 | 34.4 | 0.874 |
| - HTN | 53 | 85.9 | 49 | 54.4 | 0.652 |
| Variables | Ibuprofen Group (N = 90) | Control Group (N = 90) | P-value | ||
|---|---|---|---|---|---|
| No | % | No | % | ||
| Baseline dyspnea (%) | 12 | 13.3 | 10 | 11.1 | 0.821 |
| Baseline fever (%) | 69 | 76.7 | 71 | 78.9 | 0.858 |
| Baseline cough (%) | 46 | 51.1 | 49 | 54.4 | 0.765 |
| Baseline lymphopenia (%) | 35 | 38.9 | 33 | 36.7 | 0.878 |
| Positive CT chest finding | 70 | 77.8 | 72 | 80 | 0.855 |
| SPO2 Mean (SD) | 94.23 (0.995) | 94.43 (1.028) | 0.187 | ||
| PO2/FIO2 ratio Mean (SD) | 301.12 (15.609) | 300.71 (15.310) | 0.859 | ||
| Variables | Ibuprofen Group (N = 90) | Control Group (N = 90) | P-value | ||
|---|---|---|---|---|---|
| No | % | No | % | ||
| Dyspnea (%) | 3 | 3.3 | 4 | 4.4 | 1.000 |
| Fever (%) | 8 | 8.9 | 14 | 15.6 | 0.255 |
| Cough (%) | 20 | 22.2 | 34 | 37.8 | 0.034* |
| Lymphopenia (%) | 13 | 14.4 | 25 | 27.8 | 0.044* |
| Need for Oxygen Supply (%) | 10 | 11.1 | 21 | 23.3 | 0.047* |
| Transfer to ICU (%) | 10 | 11.1 | 21 | 23.3 | 0.047* |
| SPO2 Mean (SD) | 96.62 (0.967) | 96.38 (0.990) | 0.095 | ||
| PO2/FIO2 Ratio Mean (SD) | 333.78 (6.784) | 334.19 (7.583) | 0.702 | ||
| Duration of Hospitazation Mean (SD) | 5.94 ± 4.582 | 8.06 ± 6.537 | 0.013* | ||
| Variables | Ibuprofen Group (N = 90) | Control Group (N = 90) | P-value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Baseline S. Ferritin (ng/ml) | 245.13 | 51.681 | 250.02 | 48.424 | 0.513 |
| Baseline S.IL-6 (pg/ml) | 14.851 | 2.3399 | 15.144 | 2.6906 | 0.436 |
| Baseline S.CRP (mg/l) | 12.916 | 3.1422 | 13.243 | 2.6776 | 0.452 |
| Measured values on day 4 | |||||
| S. Ferritin (ng/ml) | 169.11 | 22.819 | 171.04 | 22.791 | 0.570 |
| S.IL-6 (pg/ml) | 10.144 | 1.0547 | 10.054 | 1.1235 | 0.580 |
| S.CRP (mg/l) | 8.443 | 2.9997 | 8.817 | 2.9553 | 0.401 |
| Variables | Ibuprofen Group (N = 90) | Control Group (N = 90) | P-value | ||
|---|---|---|---|---|---|
| No | % | No | % | ||
| Nausea (%) | 9 | 10 | 10 | 11 | 1.000 |
| vomiting (%) | 7 | 7.8 | 6 | 6.7 | 1.000 |
| Renal impairment (%) | 1 | 1.1 | 1 | 1.1 | 1.000 |
This study contributed to the literature in terms of evaluating the efficacy and safety of early use of ibuprofen to attenuate the severity of COVID-19 and improve outcomes.
Our results showed that ibuprofen resulted in attenuation of COVID-19 symptoms, mainly cough, compared to the control group. A significant reduction in hospital length (5.94 ± 4.582 versus 8.06 ± 6.537) and incidence of ICU admission (10 patients versus 21 patients) was observed. Our results were consistent with a case series study carried out by Mina T. Kelleni [7] on 38 confirmed and highly suspected COVID-19 patients. All patients received a short 5-day regimen of NSAIDs / nitazoxanide/ azithromycin +/- cefoperazone either in full or in part. Most of the patients who were treated early fully recovered during the five days; the leucocytic/lymphocytic count was significantly improved for those with prior leucopenia or leukocytosis /lymphopenia.
Qin [8] and Bhargava [9] and their coworkers observed higher serum levels of pro-inflammatory cytokines (TNF-α, IL-1β, IL-2, and IL-6) and chemokines (IL-8) in many patients with severe COVID-19 compared to individuals with mild disease, as observed in our baseline measured (CRP, IL-6, Ferritin) in both groups
In our study, we observed a significant decline in serum inflammatory marker (CRP-IL-6-S ferritin) in the ibuprofen group ((8.4,10,169.1) compared to the baseline value (12.9,14.8,245), which was numerically lower in the ibuprofen group than the control group (8.8, 10.0, 171); however, the difference was not statistically significant (mean (SD) = 7.48 (7.6) in IG versus 8.39 (7.07) in control, p = 0.655) (Table 4). We observed a statistically significant improvement in a number of patients with lymphopenia (IG 13 versus 25 in CG with P-value = 0.044*), denoting the immunological effect of ibuprofen over the course of the disease.
This is demonstrated by Hashimoto et al. [10] in a mouse model of SARS-CoV-2 infection, which showed that NSAID treatment reduced the production of proinflammatory cytokines and impaired the humoral immune response to SARS-CoV-2, as demonstrated by reduced neutralizing antibody titers. Their findings indicated that NSAID treatment might influence COVID-19 outcomes by reducing the inflammatory response and production of protective antibodies rather than modifying susceptibility to infection or viral replication.
Although corticosteroids could be used acutely to target cytokine storms, their use in respiratory viral infection is associated with increased mortality, increased risk of secondary bacterial or fungal infections, and prolonged ICU admission [2].
There was a statistically significant (p-value 0.047) reduction in the number of patients transferred to ICU (10 versus 22 in the ibuprofen and control groups, respectively), denoting successful attenuation of manifestation of the disease. Furthermore, ibuprofen was associated with a significant reduction in hospital length of stay (5.94 ± 4.582 versus 8.06 ± 6.537 in the ibuprofen and control groups, respectively).
Considering the safety of using ibuprofen in COVID-19, our results showed insignificant and negligible side effects occurred in ibuprofen versus the control group like nausea, vomiting, and renal impairment.
Early in the pandemic, it was suggested that pre-existing use of non-steroidal anti-inflammatory drugs (NSAIDs) could lead to increased disease severity in patients with COVID-19. However, recent studies have found no associations between NSAID use, admission to the hospital, or worse outcomes for patients with COVID-19 [11].
Drake et al. [11] carried out a prospective, multicenter cohort study on 78 674 patients across 255 healthcare facilities in England, Scotland, and Wales. In this cohort, 4211 (5·8%) patients were recorded as taking systemic NSAIDs before hospital admission. Following propensity score matching, balanced groups of NSAID users and NSAID non-users were included (4205 patients in each group). At hospital admission, no significant differences were reported in severity between exposure groups. After adjusting for explanatory variables, NSAID use was not associated with worse in-hospital mortality (matched OR 0·95, 95% CI 0·84 – 1·07; p 0·35), critical care admission (1·01, 0·87 – 1·17; p 0·89), necessity for invasive ventilation (0·96, 0·80 – 1·17; p 0·69), necessity for non-invasive ventilation (1·12, 0·96–1·32; p 0·14), necessity for oxygen (1·00, 0· 89 –1·12; p 0·97), or incidence of acute kidney injury (1·08, 0·92 – 1·26; p 0·33). It was concluded that NSAID use is not linked with higher mortality or increased severity of COVID-19. Policymakers should consider reviewing issued advice regarding NSAID prescribing and COVID-19 severity.
4.1. Study Limitation
Our study has limitations. Firstly, we examined only those with moderate disease, so no conclusions can be extrapolated into other clinical groups. Secondly, we enrolled non-vaccinated patients; however, due to COVID-19 constraints, this study was conducted by the time of the 4th wave in Egypt, so the impact of natural immunity from previous infection was unknown.
We were unable to study more specific markers for cytokine storm, such as TNF-α, IL-1β, and IL-2, due to the unavailability of their kits.
Unfortunately, there was no available method to detect variant(s) of the SARS-CoV-2, which may affect the course of diseases in terms of morbidity and mortality.
CONCLUSION
It can be concluded that early use of ibuprofen as adjuvant therapy in COVID-19 management is effective and safe to attenuate the severity of diseases and improve outcomes.
LIST OF ABBREVIATIONS
| NSAIDs | = Non-steroidal Anti-inflammatory Drugs |
| CRP | = C-Reactive Protein |
| IG | = Ibuprofen |
| CG | = Paracetamol |
| SBP | = Systolic Blood Pressure |
| DBP | = Diastolic Blood Pressure |
| MAP | = Mean Arterial Pressure |
| HR | = Heart Rate |
| RR | = Respiratory Rate |
AUTHORS’ CONTRIBUTION
Amr S has full access to all the data in the study and takes responsibility for the integrity of the data. Marwa EA, Lobna S, and Ahmed M A designed and conceptualized the study. Marwa EA., Ahmed M A, and Mohamed k acquired the data. Ahmed MA and Lobna S analysed and interpreted the data. Mohmed k and Sameh R drafted the manuscript. Lobna S, Ahmed M A, and Amr S critically revised the manuscript for intellectual content.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study obtained ethical approval from the Research Ethics Committee, Faculty of Medicine, Ain Shams University, Egypt, FAMASU R 07/2022.
HUMAN AND ANIMAL RIGHTS
The study was organized and operated according to guidelines of the International Council on Harmonization (ICH) and the Islamic Organization of Medical Sciences (IOMS), the United States Office for Human Research Protection, and the United States Code of Federal Regulations, which operates under federal-wide assurance no. FWA000017585.
CONSENT FOR PUBLICATION
Written consent was taken from the patients or the next of kin.
AVAILABILITY OF DATA AND MATERIAL
The author confirm that all the data and supportive information are provided within the article.
STANDARDS OF REPORTING
CONSORT guidelines and methodology were followed.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank all their colleagues at the Ain Shams University Hospitals' Intensive Care Units and Giza Chest Hospital, who performed extensive data entry.
SUPPLEMENTARY MATERIALS
Supplementary material is available on the Publisher’s website.


