All published articles of this journal are available on ScienceDirect.
Effect of Ketamine versus Dexmedetomidine on Release of Inflammatory Mediators in Laparoscopic Hysterectomy. A Randomized Trial
Abstract
Background:
Surgery and anesthesia are sources of patients' stress and release of inflammatory mediators that have adverse effects on wound healing and remote organs.
Objectives:
To compare the effects of dexmedetomidine and ketamine on perioperative serum levels of inflammatory biomarkers (interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP).
Methods:
We included 75 patients aged 30-60, ASA I and II, and scheduled for laparoscopic hysterectomy. Randomized patients received either intraoperative ketamine (bolus dose 0.25mg/kg then continuous infusion of 250µg/kg/h), dexmedetomidine (1µg/kg bolus dose then continuous infusion of 0.5µg/kg/h), or placebo. The primary outcome was to measure perioperative inflammatory biomarkers. Hemodynamic parameters, Recovery time, and complications were secondary outcomes.
Results:
At 6 and 24 hours, IL-6 significantly increased in the control group versus ketamine and dexmedetomidine groups (113.4±14.1,107.4±13.7;50.1± 8.1,48.2± 8.1;47.7±7.1, 46.01±7.1;p<0.001). Similarly, At 6 and 24 hours, TNF-α significantly increased in the control group versus ketamine and dexmedetomidine groups (81.8±18.6,72.7±16.4; 40.6±7.1, 39.2±6.9;41.6± 7.6,39.9±7.6;p<0.001).The same for CRP (17.4±3.6,40.0±6.0;10.2±1.3,16.2± 1.2;10.9±1.8,16.3±1.9;p<0.001). Regarding hemodynamic parameters, there were significant increases in the ketamine group and decreases in the dexmedetomidine group compared to baseline. Recovery time was significantly longer in the ketamine group than in the control and dexmedetomidine group (24.3±6.4,12.6±2.0,13.5±3.3 min, respectively; P<0.001). There were no significant differences between the three groups regarding agitation, nausea, and vomiting (P=1,0.126,0.776, respectively).
Conclusion:
Both dexmedetomidine and ketamine could attenuate the inflammatory response. However, dexmedetomidine has a shorter recovery time.
Trial Registry No
Trial registry at Pan African Clinical Trials Registry.
The number is (PACTR201910617459894: date of registration 10/24/2019).
1. INTRODUCTION
In gynecological procedures, anesthesia and surgery are sources of stress to the patients. The nervous, endocrine, and immune systems are all involved in the reactions to this stress, including cytokine secretion, which results in an inflammatory response [1, 2]. The minimally invasive endoscopy does not eliminate the problem [3].
One of the strategies used to reduce systemic inflammatory response is to use an anesthetic or sub-anesthetic dose of general anesthesia or opioid with a potential anti-inflammatory effect. The effect of ketamine on perioperative inflammatory response has been widely studied [4]. Recently, dexmedetomidine has been suggested to have anti-inflammatory properties [5]. It was discussed in vitro studies, where one showed the anti-inflammatory effect of dexmedetomidine after induced spinal cord injury in rats without clarifying the exact mechanism. Moreover, after induced ischemic perfusion injury, dexmedetomidine shows a neuroprotective effect on the hippocampus and dentate gyrus of rats. Another study reported the importance of early administration of dexmedetomidine to gain its anti-inflammatory effect after endotoxin injection in rats, which is a dose-dependent effect, and this suggests the benefit of dexmedetomidine during sepsis [6-8]. A few human studies on patients in the ICU reported that dexmedetomidine, when used for sedation, attenuates the inflammatory effects of sepsis. For mechanically ventilated patients postoperative, it reduces the inflammatory reaction to surgical trauma with a slight effect on adrenocortical function [9, 10].
Our study aims to evaluate the effect of intraoperative dexmedetomidine, or ketamine, on the release of inflammatory biomarkers, including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and C-reactive protein (CRP) in patients undergoing laparoscopic hysterectomy. Moreover, we evaluate the safety profile of both drugs.
2. METHODS
After obtaining the approval of the institutional ethics committee and trial registry, this prospective double-blinded randomized study was carried out from November 2019 to May 2020. The trial followed the CONSORT 2010 statement guidelines for conducting a randomized controlled trial, and written informed consent was obtained from all subjects participating in the trial.
Seventy-five female patients, aged 30-60, American Society of Anesthesiologists (ASA) physical status Classification I– II, and scheduled for elective laparoscopic hysterectomy were included in the study.
The exclusion criteria included patient refusal, severe respiratory or cardiac disorders, hepatic or renal insufficiency, allergy to any of the study drugs, body mass index >35 kg /m2, uncontrolled diabetes, autoimmune diseases, and use of any drug that might affect the immunity as chemotherapy or hormonal treatment.
Patients were randomized into three equal groups (25 patients each at a 1:1 allocation ratio) using computer-generated random numbers concealed in sealed opaque envelopes indicating the group of assignment. A blinded nurse who did not participate in the study or data collection made participants enroll. In group C (control group), patients received general anesthesia (GA) only, while in group K (ketamine group) received racemic ketamine, and those in group D (dexmedetomidine group) received dexmedetomidine.
One anesthetist conducted GA, and another one collected the data (without being informed by the group assignment). In addition, patients and nurses were blinded to the group assignment.
In the preoperative preparatory room, an intravenous line was established; midazolam 2 mg intravenous was given to all patients. A venous blood sample was withdrawn to determine baseline values of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and C- C-reactive protein (CRP), which were used as inflammatory biomarkers.
On arrival at the operating theatre, monitoring was applied to all participants, including an electrocardiogram (ECG), non-invasive blood pressure (NIBP), and pulse oximetry (Cardiocaps/5; DatexOhmeda, Helsinki, Finland). Baseline values of heart rate (HR) and mean arterial pressure (MAP) were recorded.
For all patients, GA was induced with fentanyl 1µg /kg, propofol 2mg/kg, and cisatracurium 0.15 mg/kg. After endotracheal intubation, capnography and electrodes for monitoring the Bispectral Index (BIS) were added to the previous monitoring. Patients in group K received racemic ketamine 0.25 mg/kg as an intravenous bolus dose over 10 min and then intravenous infusion at a rate of 250 µg · kg-1 · h-1, While patients in group D received dexmedetomidine 1 µg/kg as an intravenous bolus dose over 10 min, and then intravenous infusion at a rate of 0.5 µg · kg-1 · h-1.
A bolus dose of either drug was given before the skin incision, followed by continuous infusion according to the group of assignment. Anesthesia was maintained by isoflurane 1.5-2% in 50% oxygen to keep the BIS within 40 and 60, and cisatracurium 0.03 mg/kg as required to keep a train-of-four (TOF) count of one and ventilator settings were adjusted to keep EtCO2 between 35 and 40 mmHg. Anesthesia and drug infusion were discontinued at the beginning of the skin suture.
After completion of the surgical procedure, fentanyl 1 µg/kg and ondansetron 4 mg intravenous were given. Tracheal extubation was done after reversal of the neuromuscular block using neostigmine 2.5 mg and atropine 1 mg and fulfillment of extubation criteria (full consciousness, hemodynamic stability, and adequate reversal of neuromuscular block guided by TOF). All patients received 1 mg paracetamol/6h and 30 mg ketorolac/8h (according to our institutional standard protocol of postoperative analgesia).
All patients were transferred to the post-anesthesia care unit (PACU), and they were transferred to the surgical ward according to the modified Aldrete score (if the score is ≥9).
Our primary outcome was to assess the inflammatory biomarkers TNF-α, IL-6, and CRP at the following points: T1; baseline before induction of anesthesia; T2; 6 hours after injection of the study drug; T3; 24 hours after injection of the study drug.
Venous blood samples were collected using EDTA tubes. Samples were immediately placed on ice and centrifuged at 2000 g for 10 minutes. Then, the plasma was frozen and stored at −70 °C until further analyses, which were performed within 10 days after collection. The sandwich ELISA (enzyme-linked immunosorbent assay) method using commercially available tests (Quantikine ELISA Kit, R&D Systems, Minneapolis, USA) was applied for the determination of TNF-α, IL-6, and CRP levels. The absorbance measurements were performed using a photometer for microplate Elx 800 by Bio-Tek Instruments (USA). The absorbance values were read for the wavelength of λ = 450 nm with Λ correction 540 or 570 nm. The absorbance was converted into concentration units based on a standard curve.
Secondary measurements were MAP and HR, which were measured at the following points: baseline before induction of anesthesia (T0), immediately after intubation (T1), immediately after the end of the bolus dose of the study drug (T2), then every 10 min until the end of surgery. Recovery time was measured from discontinuation of anesthesia and reversal of muscle relaxant until transfer to PACU. Any adverse effects like postoperative nausea and vomiting (PONV), Hypotension (MAP < 65 mmHg), bradycardia (HR< 50 beats/min), and psychomimetic changes such as agitation, hallucinations or vivid dreams up to 24 h after surgery) were recorded.
The sample size was calculated based on a previous study [11]. We hypothesized that the attenuation in cytokine concentration would be at least 30% 6 h after intravenous dexmedetomidine infusion intraoperative. Therefore, twenty-one patients are required in each group at an alpha value of 0.05 and 90% power of the study. To overcome the dropped-out cases, 25 patients are included in each group.
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp). The Kolmogorov- Smirnov test was used to verify the normality of the distribution of variables; comparisons between groups for categorical variables were assessed using the Chi-square test (Fisher or Monte Carlo). ANOVA was used to compare more than two groups for normally distributed quantitative variables and was followed by a Post-Hoc test (Tukey) for pairwise comparison. ANOVA with repeated measures and Post-Hoc test (adjusted Bonferroni) was assessed for comparison between different periods; the results' significance was judged at the 5% level.
3. RESULTS
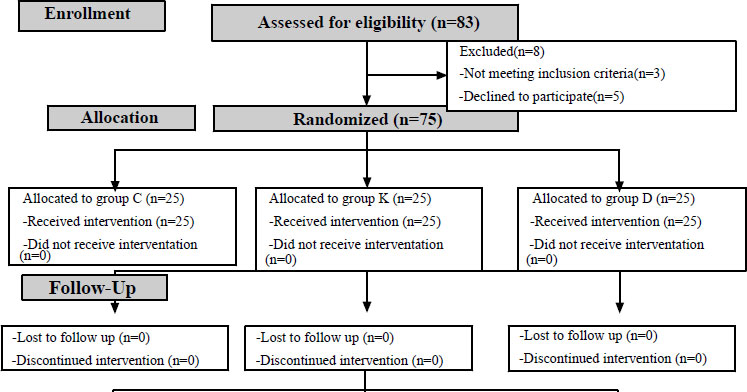
Over six months, 83 patients were assessed for eligibility, but only 75 patients were eligible for the analysis. Three patients did not meet the inclusion criteria, and five patients refused to participate in the study. No patients were lost during the follow-up. The patients were randomly allocated to one of the three groups. Finally, 25 patients in each group were analyzed, as shown in the CONSORT flow diagram (Fig. 1). The study groups were comparable in regard to age, body mass index, and duration of surgery (Table 1).
| Parameter |
Control (n = 25) |
Ketamine (n=25) |
Dexmedetomidine (n = 25) |
P |
|---|---|---|---|---|
| Age (years) | 45.2 ± 8.7 | 45.7 ± 8.9 | 45.0 ± 8.6 | 0.964 |
| BMI (kg/m2) | 29.6 ± 3 | 28.8 ± 2.8 | 28.6 ± 2.5 | 0.352 |
| Duration (min) | 139.4 ± 14.1 | 137.4 ± 12.9 | 140.2 ± 11.7 | 0.734 |
| Recovery time (min) | 12.6b ± 2.0 | 24.3a ± 6.4 | 13.5b ± 3.3 | <0.001* |
|
Inflammatory Biomarkers |
Control (n = 25) |
Ketamine (n=25) |
Dexmedetomidine (n = 25) |
P |
|---|---|---|---|---|
| IL-6 (pg/ml) | ||||
| Baseline | 45.5 ± 8.5 | 45.7 ± 8 | 43.9 ± 7.1 | 0.664 |
| 6 hour | 113.4# ± 14.1 | 50.1a# ± 8.1 | 47.7a# ± 7.1 | <0.001* |
| 24 hour | 107.4# ± 13.7 | 48.2a# ± 8.1 | 46.01a# ± 7.1 | <0.001* |
| TNF-α (pg/ml) | ||||
| Baseline | 38.2 ± 8.7 | 37 ± 6.8 | 37.33 ± 7.7 | 0.835 |
| 6 hour | 81.8# ± 18.6 | 40.6a# ± 7.1 | 41.6 a# ± 7.6 | <0.001* |
| 24 hour | 72.7# ± 16.4 | 39.2a# ± 6.9 | 39.9a# ± 7.6 | <0.001* |
| CRP (mg/L) | ||||
| Baseline | 5.0 ± 1.04 | 4.7 ± 0.5 | 4.9 ± 1.04 | 0.450 |
| 6 hour | 17.4# ± 3.6 | 10.2a# ± 1.3 | 10.9a# ± 1.8 | <0.001* |
| 24 hour | 40.0# ± 6.0 | 16.2a# ± 1.2 | 16.3a# ± 1.9 | <0.001* |
While there were significant increases in the concentrations of inflammatory biomarkers (TNF-α, IL-6, and CRP) 6 and 24 h postoperative in the 3 studied groups, there were significant differences between the ketamine group and dexmedetomidine group compared to the control group with no significant differences between ketamine group and dexmedetomidine group. (Table 2).
| Adverse Effects |
Control (n = 25) |
Ketamine (n=25) |
Dexmedetomidine (n = 25) |
P |
|---|---|---|---|---|
| Agitation | 0(0.0%) | 1(4.0%) | 0(0.0%) | 1.000 |
| Nausea | 2(8.0%) | 6(24.0%) | 1(4.0%) | 0.126 |
| Vomiting | 1(4.0%) | 2(8.0%) | 0(0.0%) | 0.776 |
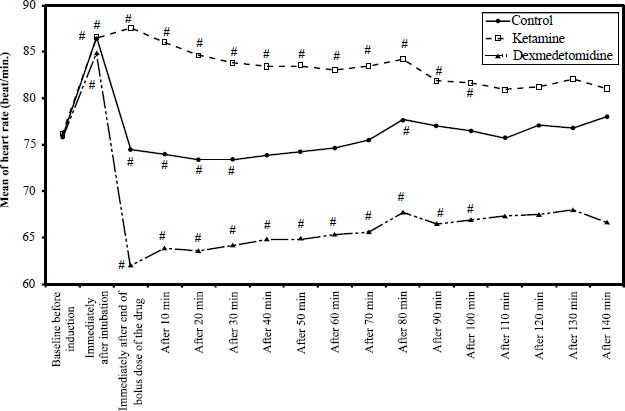
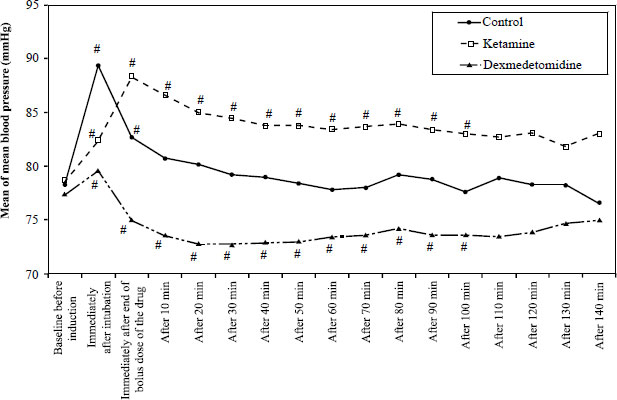
It has been found that hemodynamic parameters (HR and MAP) were significantly increased compared to the baseline values in the ketamine group. In contrast, in the dexmedetomidine group, hemodynamic parameters were significantly decreased compared to the baseline values. The patients were clinically stable and did not need any intervention. The overall measurement results are summarized in Figs. (2 and 3).
The recovery time was significantly prolonged in the ketamine group (24.3 ± 6.4 min) compared to the control group (12.6 ± 2.0 min) and dexmedetomidine group (13.5 ± 3.3 min) (Table 1).
The results showed that there were no statistically significant differences between the studied groups regarding the adverse effects. (Table 3).
4. DISCUSSION
Our study showed that both ketamine and dexmedetomidine had a favorable impact on postoperative inflammatory response. There was delayed recovery in patients who received ketamine with more clinical side effects; nevertheless, it did not reach statistical significance. Both ketamine and dexmedetomidine affected the hemodynamic parameters statistically. However, there was no clinical significance that patients were stable and did not receive intervention.
Dexmedetomidine is a 2-adrenergic agonist which has an analgesic, sedative, and sympatholytic effect. Few human studies on patients in ICU [9, 10] demonstrated the anti-inflammatory properties of dexmedetomidine.
To our knowledge, it is the first study to compare the effect of dexmedetomidine and ketamine on the suppression of inflammatory response (release of inflammatory biomarkers, including TNF-α, IL-6, and CRP) in female patients undergoing laparoscopic hysterectomy. In addition, we assessed the side effects of both drugs.
The choice of TNF-α and IL-6 to be assessed was due to their very early involvement in the initiation of the inflammatory response, called acute phase reactants [12]. In addition, IL-6 is not affected by anesthetic intervention alone, so it reflects the surgery-induced stress [13].
We found that dexmedetomidine was able to mitigate the early postoperative inflammatory response. Also, earlier studies reported that dexmedetomidine alleviated inflammation in a rat model with spinal cord injury [6]. Furthermore, it significantly decreased the development of postoperative intraabdominal adhesions in rats with abdominal surgery, which suggested the antioxidant and anti-inflammatory effects of dexmedetomidine [14]. This anti-inflammatory effect of dexmedetomidine was also demonstrated in clinical trials, where its administration postoperatively to patients requiring sedation and mechanical ventilation was associated with decreased concentrations of IL-6 levels [10]. Dexmedetomidine was reported also to decrease IL-1, TNF-α, and IL-6 concentrations in patients with sepsis [15].
Few studies have evaluated the anti-inflammatory effects of dexmedetomidine on surgery-induced inflammatory response, whose results were consistent with our study. Kang et al. [1] found that patients undergoing laparoscopic cholecystectomy who received dexmedetomidine had significantly lower concentrations of IL-1β, TNF-α, IL-10, and CRP with lower leukocytic count compared to the control participants. Ueki et al. [5] demonstrated that dexmedetomidine reduced the increase in postoperative IL-6 levels in patients undergoing cardiopulmonary bypass. Ding et al. [16] found dexmedetomidine to significantly decrease TNF-α and IL-6 compared to the control group in elderly patients undergoing laparoscopic radical prostatectomy. Li et al. [17] reported that dexmedetomidine significantly reduced the intraoperative and postoperative levels of TNF-α and IL-6 in patients undergoing open gastrectomy. Jiang et al. [18] stated that dexmedetomidine had significantly decreased IL-6 levels after open esophagectomy.
The mechanism of the anti-inflammatory effect of dexmedetomidine has yet to be completely understood. Dexmedetomidine was postulated to modify the production of cytokines by macrophages and monocytes [19]. In addition, it was found to possess an antiapoptotic effect, which may contribute to protection against anesthetic-induced apoptosis in vivo and in vitro [20].
Moreover, dexmedetomidine could augment the phagocytic activity of macrophages in vitro [21]. Dexmedetomidine may also suppress inflammation through a central sympatholytic effect by stimulation of the cholinergic anti-inflammatory pathway [22]. Another proposed mechanism is the inhibition of nuclear factor kappa B activation [23]. It might be possible that the antinociceptive action of dexmedetomidine contributes to its anti-inflammatory effect [24].
Ketamine is an anesthetic and analgesic drug with anti-inflammatory effects. A meta-analysis concluded that ketamine has a significant inhibitory effect on postoperative IL-6 levels and thereby mitigates the early inflammatory response after surgery [4]. Moreover, ketamine has been shown to decrease the postoperative levels of TNF-α, IL-1β, and IL-6 [25]. The current study showed that ketamine and dexmedetomidine suppressed the increase in the postoperative level of inflammatory markers with no significant differences. This finding indicates that both drugs could be equally effective in attenuation of the postoperative inflammatory response.
In our study, all patients were clinically stable, and none needed any intervention. However, HR and MAP were significantly increased in the ketamine group and significantly decreased in the dexmedetomidine group compared with the baseline values. Similar hemodynamic effects of dexmedetomidine were observed in several studies [16, 17, 26]. This is considered a myocardial protective effect of dexmedetomidine as it prevents acute increases during airway management, reduces myocardial oxygen demand, and decreases the risk of myocardial injury [27].
We found that the recovery time was slightly higher in the dexmedetomidine group (13.5 ± 3.3 min) compared to the control group (12.6 ± 2.0 min). In contrast, the recovery time was significantly longer in the ketamine group (24.3 ± 6.4 min) than in the other two groups. In partial agreement with our results, previous studies showed that dexmedetomidine, when co-administered with propofol, is associated with a longer recovery time from anesthesia [28]. The lack of significant difference in recovery time between the dexmedetomidine and control groups in our study may be attributed to the protocol that differed from other studies. Delayed recovery time with ketamine administration was reported even with sub-anesthetic doses [29].
Various adverse effects were reported with dexme- detomidine administration, including cardiovascular (e.g., bradycardia, hypertension, dysrhythmias), gastrointestinal (e.g., vomiting, dry mouth), respiratory (e.g., pleural effusion, atelectasis, pulmonary edema), and metabolic adverse effects (e.g., hyperglycemia, hypocalcemia, and acidosis). The cardiovascular effects of dexmedetomidine are related to its administration with a rapid infusion rate or with a large bolus dose [30].
As for ketamine, adverse effects were reported on the respiratory system (e.g., apnea), gastrointestinal system (e.g., PONV), and recovery from anesthesia (emergence agitation) [31].
Evaluation of the adverse effects in our study elicited no significant differences among the studied groups. However, more adverse effects were recorded in the ketamine group. This difference in adverse events may bear clinical importance despite being statistically insignificant. Dexmedetomidine has been shown to prevent emergence agitation [28] and postoperative vomiting [32] more effectively than ketamine. These findings suggest that dexmedetomidine is safe and may be preferred over ketamine for having a lower rate of adverse effects as well as maintenance of hemodynamic stability with myocardial protective effect and control of emergence agitation.
Rushuang Chen et al. [33] reported the beneficial anti-inflammatory effect of intraoperative administration of dexmedetomidine. They found that dexmedetomidine could inhibit the inflammation following intestinal surgery with improved cognitive function postoperative and significantly fewer adverse effects, e.g., PONV, shivering, and drowsiness.
The study has limitations. First, short-term follow-up of the anti-inflammatory effect of the study drugs. Second, the study did not investigate the mechanism of action of the study drugs.
Further studies are proposed to compare different doses of dexmedetomidine and ketamine to know the minimum effective dose given by either continuous infusion or single injection. Furthermore, by evaluation of more inflammatory biomarkers, the mechanism of action of dexmedetomidine could be concluded.
CONCLUSION
Dexmedetomidine and ketamine were comparable to each other as regards the attenuation of the postoperative inflammatory response. However, the recovery time of dexmedetomidine was shorter than that of ketamine, with a lower rate of adverse effects.
LIST OF ABBREVIATIONS
| PONV | = Postoperative Nausea and Vomiting |
| TNF-α | = Tumor necrosis factor-alpha |
| MAP | = Mean Arterial Pressure |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study obtained ethical approval from the research ethics committee, Faculty of Medicine, Tanta University, Egypt, approval code 33104/05/19.
HUMAN AND ANIMAL RIGHTS
No animals were used that are the basis of this study. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants of this study.
STANDARDS OF REPORTING
Consort guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available from (M.R.E) the corresponding author on special request.
FUNDING
The research is self-funded.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The article is presented as a preprint at (https://www.researchsquare.com/article/rs-90812/v1).


