All published articles of this journal are available on ScienceDirect.
The Impact of Enhanced Recovery Protocols Regarding Postoperative Nausea and Vomiting Following Sleeve Gastrectomy under Triple Prophylaxis: A Controlled Randomized Study
Abstract
Background
Following a sleeve gastrectomy, Post-Operative Nausea And Vomiting (PONV) is a typical issue. Furthermore, several prophylactic techniques have been developed, such as preventive antiemetic and accelerated Recovery After Surgery (ERAS). However, PONV has not entirely disappeared, and clinicians are still working to lower PONV incidence.
Aim
Our goal was to evaluate how adopting Enhanced Recovery Protocols (ERAS) affects Postoperative Nausea and Vomiting (PONV) in comparison to standard care protocol after Laparoscopic Sleeve Gastrectomy (LSG) while receiving triple antiemetic prophylaxis.
Objective
The objective of this study was to verify that the ERAS procedure is crucial for lowering PONV despite the use of an efficient and effective antiemetic.
Methods
This is a computer-generated randomized clinical trial. Haloperidol, dexamethasone, and ondansetron were administered to all patients undergoing elective LSG, 29 patients within an ERAS protocol, and 29 within a standard care protocol. The primary finding was the incidence of PONV within 36 hours following LSG. The time to initially administer rescue antiemetic medication, number of rescue antiemetic medication administrations, postoperative opioid consumption, oral fluid tolerance, complications, and QoR-15 questionnaire for quality of recovery were the secondary outcomes.
Results
Within the first 36 hours following LSG, the incidence of PONV in the ERAS group was 17.20%, while in the non-ERAS group, it was 51.7%, with P<0.012 and higher PONV severity (P<0.021) in the non-ERAS group. The ERAS group took a longer time (6 hours) for the first rescue antiemetic medicine than the Non-ERAS group (2 hours), with P<0.001 and significantly less number of patients (20.7%) needing rescue antiemetic, compared to the Non-ERAS group (65.5%), with P<0.001. The dosage of nalbuphine needed by the ERAS group was lower (2.7±2.8) than the non-ERAS group (19.9±6.0). Regarding the QoR-15 scores, there was a significant difference in the two groups' overall performance (P <0.001). Between the two groups, there were no significant complications following surgery.
Conclusion
This study reveals that even though triple antiemetic prophylaxis was used, the ERAS protocol had a beneficial effect on PONV when compared to the standard care approach.
1. INTRODUCTION
Globally, morbid obesity is the primary factor contributing to premature mortality. In those populations, several metabolic comorbidities can be addressed or resolved, and life expectancy can be increased when bariatric surgery is used to treat extreme obesity [1, 2]. Consequently, the global demand for bariatric surgical operations has increased significantly [3].
The success and ease of use of laparoscopic sleeve gastrectomy have led to a rise in the percentage of people undergoing bariatric surgery [4].
In surgical procedures, the incidence of PONV varies from 30% to 80%, making it a prevalent issue for patients undergoing surgery [5-7]. A history of acid reflux, gastric surgery, and stomach reduction, especially after LSG, may all make the PONV worse [8, 9]. In addition, PONV delays post-anesthesia care unit (PACU) discharge, increases healthcare costs, and leads to poor patient satisfaction [7].
For patients who have a greater risk of suffering from PONV episodes, preoperative or intraoperative adminis- tration of prophylactic antiemetics is frequently used [10]. Additionally, using a mixture of antiemetic medications that target different receptor types (opioid, histamine, cholinergic, dopaminergic, serotonin, and neurokinin) rather than a single drug is preferable [11, 12]. Previous studies in patients undergoing bariatric surgery found that PONV and the requirement for rescue antiemetic medications were reduced when haloperidol, dexa- methasone, and ondansetron were combined [13].
After bariatric surgery, PONV typically continues and has a detrimental influence on patient satisfaction, hospital stay, and readmission risk, even if various combinations of perioperative antiemetics can minimize PONV.
The ERAS protocol combined with total intravenous anesthetic, multimodal analgesia, and regional anesthetic techniques can lower rates of morbidity and further lower the risk of PONV [14].
The goal of this observational, randomized trial is to examine the effects of ERAS protocol against standard care protocol adoption on PONV in patients undergoing LSG and receiving triple antiemetic prophylaxis.
2. PATIENTS AND METHODS
2.1. Permission from Ethics Authorities and Informed Consent
This clinical trial was approved by the Minia University ethical committee No. 820:6/2023 on July 1, 2023, and conducted in Minia University Hospital. It was registered at clinicaltrials.gov ID: NCT05996887 (https://clinical trials.gov/search?cond=NCT05996887) before patient enrollment.
This prospective, controlled, observational study was created, and the original protocol was followed during the trial. It can be obtained upon request. All study participants provided written informed consent, which was collected by the primary investigator or research team members who underwent consent training. During the meeting with possible participants, the goals, procedures, expected advantages, and hazards were explained. Throughout the study, patients were allowed to revoke their permission at any moment.
2.2. Participant Eligibility Criteria
Patients of both sexes aged 18 to 65, with American Society of Anesthesiologists II and III and a body mass index between 40 and 60 kg/m2 undergoing elective Sleeve Gastrectomy, were among those who met the eligibility parameters. In comparison, the exclusion criteria included patients with psychiatric disorders, use of opioid, hormonal, and antiemetic medications twenty-four hours before the operation, patients with severe consequences during surgery (such as shock, cardiac arrest, bleeding, or needing a transfusion), or patients who were hypersensitive to or contraindicated for ondansetron, haloperidol, or dexamethasone.
2.3. Outcomes
The incidence of PONV within 36 hours following LSG was the primary outcome. The quantity of opioids consumed after surgery, the number of rescue antiemetic drug administrations, the duration to tolerate oral fluid, time to initial delivery of rescue antiemetic drug, and any complications were among the secondary outcomes. In addition, the level of recovery (questionnaire: QoR 15 patient survey) [15] was assessed. Pain, physical comfort, physical independence, psychological support, and emotional state were the five characteristics that were evaluated by the QoR-15. The process took less than three minutes to complete [15].
2.4. Randomization
A computer-generated randomization list was created by a study statistician before the start of the study, using simple randomization to assign study arm assignments in a 1:1 ratio. This list was sent to an operating room pharmacist and staff members who prepared the necessary medications and patients and allocated the patients into two groups, each containing 30 patients. A non-ERAS group in which every patient received the same standard care protocol and an ERAS group in which all patients followed the recommendation of ERAS society guidelines [16].
2.5. Blinding
The group assignment was concealed from the patients. The postoperative anesthesia care unit nurses and the anesthesia team were not blinded. The ward's care team was unaware of the group assignment, and the researcher determining the extent of PONV was blinded to the treatment.
2.6. Study Design
Prior to the procedure, the patient was questioned regarding their history of motion sickness, prior PONV episodes, and current smoking habits (simplified Apfel score) [17]. From the medical records, we gathered the following information: age, sex, height, weight, and ASA physical state, both prescribed and administered. On that particular day of the operation, a member of the study team examined the patients to determine their eligibility, received their informed permission in the preoperative area, and enrolled the participants in the trial.
2.7. Interventions
2.7.1. Antiemetic Protocol
All patients in both groups received the same antiemetic regimen (infusion of 8 mg of dexamethasone 90 minutes before anesthetic induction, 2 mg of haloperidol following anesthesia induction, and 8 mg of ondansetron 20 to 30 minutes before the conclusion of the procedure).
Following the application of standard ASA monitors (Schaumburg, Illinois) and achieving IV access, general anesthesia was administered. The corrected body weight (CBW) =[0.4 × (weight at present − ideal weight)] and the ideal body weight (IBW) = height (in cm)—100 for males and height (in cm)—105 for females, were used to determine the dosages of anesthetic medications for induction and sustaining anesthesia [13].
2.8. The Groups
2.8.1. Non- ERAS Group
Six hours prior to induction, the patients were asked to fast for food and liquids. Propofol (2 mg/kg of CBW), fentanyl (3 μg/kg of CBW), and cisatracurium (0.1 mg/kg of IBW) were used in the induction of anesthesia along with the antiemetic protocol mentioned before. Isoflurane (1% in a mixture of oxygen and air 1:1), an extra dosage of fentanyl (1 μg/kg of CBW), and cisatracurium, if needed, were used for maintenance. Neostigmine (0.04 mg/kg of IBW) and atropine (0.015 mg/kg of IBW) were used to reverse neuromuscular inhibition. The fluid used was maintained at a rate of 2 mL kg−1 h−1 of crystalloid normalized to the ideal body weight, which was calculated according to the Robinson formula (18). After anaesthesia induction, the Non-ERAS group received intravenous ketorolac (30 mg) for postoperative analgesia, which was repeated every 8 hours.; acetaminophen 1g IV was administered prior to extubation and every 6 hours postoperatively; and Nalbuphine 10mg (20mg/1ml) was administered if NRS >4, and the pain was re-assessed at 15 min intervals, and an additional 5 mg intravenous of Nalbuphine was given as needed to keep Visual Analogue Scale (VAS)<4.
2.8.2. ERAS Group
2.8.2.1. Preoperative Protocols
2.8.2.1.1. Preadmission Care
Preoperative information and instructions should be provided to all patients. It is recommended to quit smoking four weeks before surgery. In addition, preoperative weight loss with a very low or low-calorie diet ought to be advised.
2.8.2.1.2. Maintenance of Normovolemia and Optimization of Tissue Perfusion
Maintaining normovolemia and optimizing tissue perfusion and oxygenation are the goals of fluid management. So, avoid using both liberal and restrictive tactics.
2.8.2.1.3. Pain Management
Acetaminophen 1000 mg and 400 mg gabapentin oral tablets are given two hours prior to anesthesia.
2.9. Intraoperative and Postoperative Protocols
2.9.1. Patient Positioning
Improved pulmonary mechanics and gas exchange are achieved by assuming a reverse Trendelenburg position with flexed hips during pneumoperitoneum.
2.9.2. Anesthesia
Total Intravenous Anesthesia (TIVA) [16, 20] with propofol (2 mg/kg iv of CBW) and maintained with an intravenous propofol infusion (75–150 µg/kg/ h) adjusted to maintain the mean arterial pressure within ± 20% of each patient’s baseline value is recommended. Dexme- detomidine is used (0.1–0.3 µg/kg) in 100 ml of normal saline over 10 min and kept on an intravenous infusion (0.5 µg/ kg/h) until the procedure is completed; in addition, 50 ml of normal saline is used to prepare 1 mg/kg/h of lidocaine to run at flow rate 50 ml/h, with ketamine prior to incision (0.5 mg/kg iv), then (0.5 mg/kg/h) till the end of operation. For muscle relaxation and maintenance, cisatracurium 0.1 mg/kg of IBW iv. Neostigmine 0.04 mg/kg iv of IBW and atropine 0.015 mg/kg iv of IBW are used to reverse neuromuscular inhibition. Patients are ventilated using a combination of air and oxygen.
2.9.3. Fluid Management
Avoiding the strategies that are liberal or restrictive. The fluid therapy is to keep a urine output of 0.5-1 mL/kg per hour, with deliberate administration of colloid solution if needed.
2.9.5. Postoperative Analgesia
Magnesium sulfate (30 mg/kg) and maintenance infusion (10 mg/kg/h) for 24 h.; Lidocaine (1mg/kg/h) for 24 h.; Bilateral TAP block at the end of operation; Ketorolac 30 mg every 6 hr., Acetaminophen IV in a dose of 1 g every 6 h postoperative, and IV Nalbuphine 5-10mg (20mg/1ml) are recommended in order to ease breakthrough pain.
2.10. Postoperative Care for Both Groups
• The level of sedation was assessed 15 min after arrival to the PACU and after 2, 6, and 24h using the Ramsay score [21].
2.11. Data Collection and Monitoring
The incidence of PONV was assessed after 36 h using a PONV score: I =neither nausea nor vomiting, II = nausea but not vomiting, III = mild to moderate vomiting, and IV indicates severe and frequent vomiting, defined as more than five episodes in a 24-hour period [22].
The frequency of vomiting was as follows: I represents no vomiting, II represents vomiting episodes 1-2 times within 24 hours, III represents vomiting episodes 3–5 times within 24 hours, and IV represents vomiting episodes more than 5 times within 24 hours. This was used to measure the severity of postoperative vomiting (POV). A numerical rating system was used to determine the degree of postoperative nausea (PON) (I = mild, II = moderate, and III = severe) [22]. For the first two hours, nausea was evaluated hourly; for the next four hours, it was evaluated every two hours; and for the final 36 hours, it was evaluated every four hours.
Other parameters assessed included time to initial rescue antiemetic medication administration, the number of patients who needed rescue antiemetic drug, utilization of opioids after surgery, postoperative pain score at rest, the time to tolerate oral fluid, and the recovery quality evaluated using QoR-15 [15, 23] which is a survey upon release. The questionnaires were completed over the phone the following morning for patients who were released earlier than 36 hours.
2.12. Statistical Analysis
Prior to the investigation, the number of patients needed in each group was established using data from a pilot study and a power calculation. In that pilot research, 10% of the ERAS group and 45% of the control (Non-ERAS) group had vomiting. Using G Power 3.1 9.2 software, a sample size of 29 patients per group was shown to provide 80% power at the 0.05 significant level.
Data processing was done with IBM SPSS software, version 26, Chicago, IL, USA, along with checking, entering, and analyzing the data. For categorical variables, frequencies, and percentages, Fisher exact and chi-square tests were used to compare the two groups. Results were presented as mean ± standard deviation. For normally distributed data and compared using a two-sample Student’s t test. The nonparametric data were presented as median, and IQR and Mann-Whitney U-tests were used to compare medians of two independent groups. The Wilcoxon signed-rank test for nonparametric data and the paired t-test for parametric data were used for pairwise comparisons. The data were shown as mean ± standard deviation or as median (with interquartile range).
3. RESULTS
3.1. Study Protocol
Seventy-five patients were approached, and 60 patients were assigned at random to the trial. Seven patients did not meet the requirements for inclusion, and eight patients declined to participate. One patient in the non-ERAS and one in the ERAS groups was lost to follow-up at thirty-six hours due to early discharge before their postoperative care was finished. Fig. (1) represents the flow chart for patient recruitment, allocation, follow-up, and analysis.
3.2. Clinical Characteristics
Table 1 provides an overview of the clinical and demographic features of ERAS and non-ERAS patients. There were no discernible variations between the groups.
3.3. PONV Score and Severity
As shown in Fig. (2), The incidence of PONV was 17.2% (5/29) within the first 36 h after LBS in the ERAS group compared to 51.7% (15/29) in the Non-ERAS group with significant P-value =0.012. Additionally, the non-ERAS group had significantly higher PONV severity than the ERAS group P=0.021.
Regarding the severity of POV, within 0-24 hours and 24-36 hours, it showed significant increases in the non-ERAS group when compared to the ERAS group, and P<0.026 and P=0.008, respectively. In addition, the severity of nausea in the non-ERAS group showed significant increases compared to the ERAS group P<0.001 all over the 36 hours.
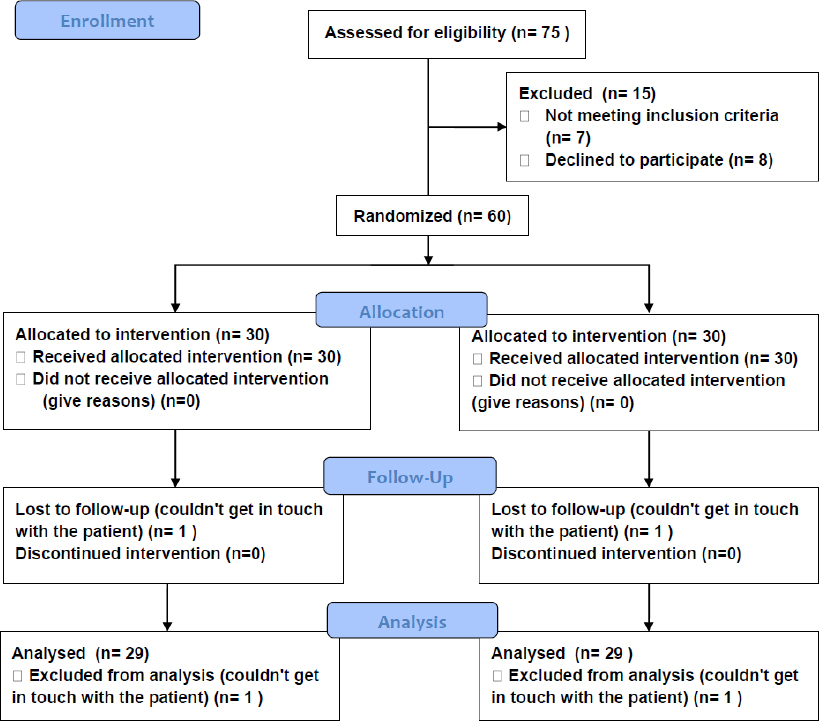
Consort flow chart.
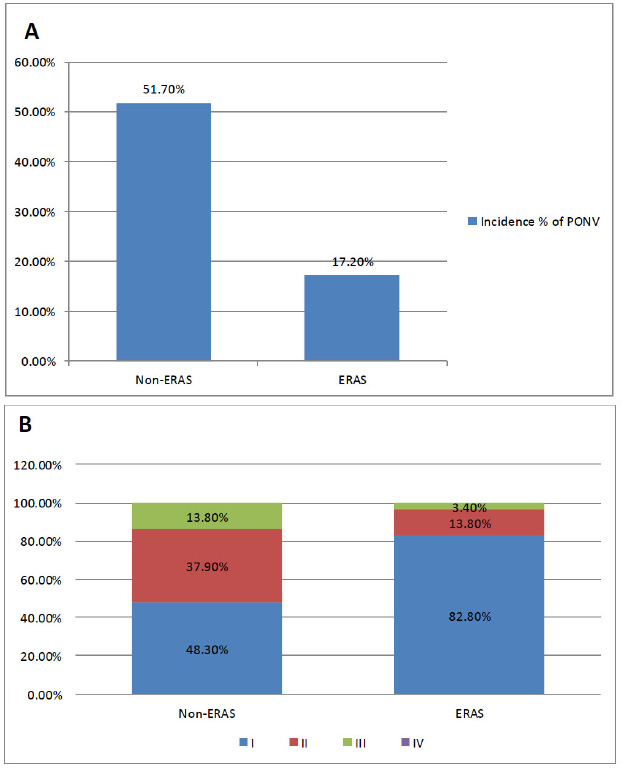
Postoperative nausea and vomiting at 36h in both groups (A & B).
A: Incidence % of PONV in the two groups
B: Percentage of the grades of PONV in the two groups; I =neither nausea nor vomiting, II = nausea but not vomiting, III = mild to moderate vomiting, and IV indicates severe and frequent vomiting, defined as more than five episodes in a 24-hour period. Pearson Chi-Square Fisher’s and Exact Test for numbers and percentages were used to analyze the variables.
*P is significant when at <0.05.
| Characteristic | Non-ERAS | ERAS | P-value |
|---|---|---|---|
| N=29 | N=29 | ||
|
Age (year)c Range Mean ± SD |
[24-36] 29.1±3.5 |
[23-36] 28.7±3.8 |
0.670 |
|
Sex n (%)c Male Female |
16(55.2%) 13(44.8%) |
16(55.2%) 13(44.8%) |
1.0 |
|
Body mass index (kg m−2)b Range Mean ± SD |
[39-52] 45.7±4.4 |
[40-52] 45.5±3.7 |
0.897 |
|
American Society of Anesthesia n (%)c II III |
19(65.5%) 10(34.5%) |
17(58.6%) 12(41.4%) |
0.066 |
|
Apfel score n (%)c 1 2 3 4 |
1(3.4%) 10(34.5%) 13(44.8%) 5(17.2%) |
1(3.4%) 8(27.6%) 14(48.3%) 6(20.7%) |
1.0 |
|
Duration of operation(min)a Range Mean ± SD |
(43-90) 62.1±12.3 |
(45-90) 64.1±12.5 |
0.536 |
aIndependent Samples T-test.
b Mann Whitey test.
cChi-square test.
3.4. Recovery Criteria in Both Groups
The ERAS group took a longer time, 6 h(4-6h), for the first rescue antiemetic medicine than the non-ERAS group, which took 2h(1-4h), with a significant P-value of <0.001. The number of patients requiring rescue antiemetics were also significantly less in the ERAS group, which showed 6(20.7%) compared to the non-ERAS group, which showed 19(65.5%) with P <0.001. Regarding postoperative opioid consumption, the ERAS group required smaller amounts of nalbuphine (2.7±2.8 mg) than the non-ERAS group (19.9±6.0 mg), as only fifteen patients (51.7%) required nalbuphine once. In contrast, in the other group, all patients received nalbuphine P <0.001. The patients in the ERAS group tolerated oral fluids more rapidly than the non-ERAS group, with P <0.001 (Table 2).
The sedation score showed significant elevation in the ERAS group compared to the non-ERAS group at 15 minutes and 2h after surgery, with P = 0.002 and P <0.001, respectively (Table 3).
| Severity | Non-ERAS | ERAS | P-value |
|---|---|---|---|
| N=29 | N=29 | ||
|
POV, 0-24 h n (%) I II III IV |
14(48.3%) 10(34.5%) 5 (17.2%) 0(0%) |
23(79.3%) 6(20.7%) 0(0%) 0(0%) |
0.026* |
|
POV, 24-36 h n (%) I II III IV |
17(58.6%) 10(34.5%) 2(6.9%) 0(0%) |
27(93.1%) 2(6.9%) 0(%) 0(%) |
0.008* |
|
PON, 0-2 H n (%) I II III |
0(0%) 13(44.8%) 16(55.2%) |
21(72.4%) 8(27.6%) 0(%) |
<0.001* |
|
PON, 2-12 H n (%) I II III |
5(17.2%) 15(51.7%) 9(31.0%) |
23(79.3%) 6(20.7%) 0(0%) |
<0.001* |
|
PON, 12-24 H n (%) I II III |
9(31.0%) 15(51.7%) 5(17.2%) |
26(89.7%) 3(10.3%) 0(0%) |
<0.001* |
|
PON, 24-36H n (%) I II III |
13(44.8%) 13(44.8%) 3(10.3%) |
27(93.1%) 2(6.9%) (0%) |
<0.001* |
Pearson Chi-Square Fisher’s and Exact Test for numbers and percentages were used to analyze the variables.
*P is significant when at <0.05.
| Variables | Non-ERAS | ERAS | P-value |
|---|---|---|---|
| N=29 | N=29 | ||
|
Time of first rescue antiemetic drug (h)b Median(IQR) |
2(1-4) | 6(4-6) | 0.002* |
|
Number of patients needed rescue antiemetic, c n (%) |
19(65.5%) | 6(20.7%) | <0.001* |
|
Postoperative opioid consumption(mg) a Range Mean ± SD |
[10-30] 19.9±6.0 |
(0-10) 2.7±2.8 |
<0.001* |
| Number of patients needed rescue opioid,c n (%) | 29(100%) | 15(51.7%) | <0.001* |
|
Ramsay Sedation score,Median(IQR)b After 15 min After 2h After 6h After 24h |
3(1-3) 2(1-3) 2(1-3) 2(1-2) |
3(2-5) 3(1-3) 2(1-3) 1(1-3) |
0.002*
<0.001* 0.203 0.376 |
|
The time to tolerate oral fluids(h)a Range Mean ± SD |
(4-8.5) 5.9±1.3 |
(1.5-7) 4±1.4 |
<0.001* |
aIndependent Samples T-test.
bMann Whitey test.
cChi-square test.
*Significant level at P-value < 0.05.
| Variables | Non-ERAS | ERAS | P-value |
|---|---|---|---|
| N=29 | N=29 | ||
| PART A | |||
| 1. Breathing | 6(5-8) | 8(6-9) | <0.001* |
| 2. Food | 5(4-7) | 7(6-8) | <0.001* |
| 3. Rest | 6(5-7) | 7(6-9) | <0.001* |
| 4. Sleep | 6(5-7) | 8(7-9) | <0.001* |
| 5. Hygiene | 5(4-6) | 6(5-7) | <0.001* |
| 6. Communication | 5(4-7) | 7(6-8) | <0.001* |
| 7. Support | 5(4-7) | 7(5-8) | <0.001* |
| 8. Return to work | 5(4-7) | 7(6-8) | <0.001* |
| 9. Feeling in control | 4(3-6) | 8(6-9) | <0.001* |
| 10. Well-being | 6(5-7) | 8(6-9) | <0.001* |
| PART B | |||
| 11. Moderate pain | 5(4-6) | 7(6-8) | <0.001* |
| 12. Severe pain | 7(6-8) | 8(7-9) | <0.001* |
| 13. Nausea/vomiting | 5(4-7) | 7(5-9) | <0.001* |
| 14. Anxiety | 6(4-7) | 7(5-8) | <0.001* |
| 15. Depressed | 8(6-9) | 8(7-9) | 0.178 |
| Total | 85(80-89) | 109(102-113) | <0.001* |
Mann Whitey test was used to analyze the variables. Values are given as median, (IQR)
P-value < 0.05 is considered significant.
PART A:Over the past day, how have you been feeling? (0 to 10), where 10 is all of the time (great) and 0 is never (bad).
PART B:Which of the following have you experienced in the past 24 hours? (10 to 0, where 0 represents all of the time (bad) and 10 represents none of the time (great). 1=easy breathing; 2=able to eat; 3=feeling rested; 4=having slept well; 5=able to take care of one's personal hygiene and toiletries without assistance; 6=able to speak with family or friends; 7=receiving support from hospital staff; 8=able to resume work or regular household activities; 9=feeling at ease and in control; 10=feeling generally well-being;11 denotes mild pain, 12 severe pain, 13 nausea or vomiting, 14 feeling nervous or uneasy, and 15 dejected or melancholy.
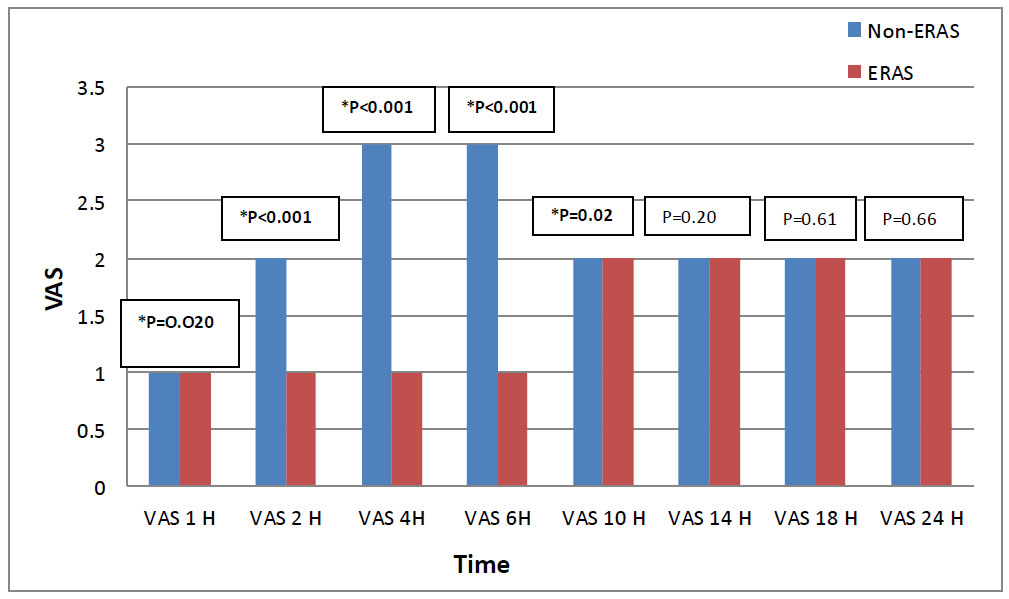
Visual analogue scale (VAS) in both groups.
Mann Whitey test used to analyze the variables; values are given as median, (IQR).
*: Significant level at P-value < 0.05.
Pain assessment in both groups showed a significant elevation of VAS score in the Non-ERAS group as early as 1 hour postoperative, while in the ERAS group, the VAS score started to elevate at 10h postoperative. Further- more, Compared to the Non-ERAS group, the ERAS group's VAS score (median and interquartile range) was lower at 1h, 2h, 4h, 6h, and 10h postoperative P= 0.020, P<0.001, P<0.001, P<0.001, and P= 0.020, respectively (Fig. 3).
Between the two groups, there was a significant difference in the overall QoR-15 scores for patients who had a good or poor postoperative recovery P <0.001. The median [range] QoR-15 scores at 36 h were 109(102-113) for good recovery in the ERAS group vs 85(80-89) for poor recovery in the non-ERAS group with P <0.001 (Table 4).
The MAP and HR showed significant decreases in both groups at all times compared to the baseline readings. However, the non-ERAS group showed significant elevations in HR all the time after 5 min intraoperatively and postoperative compared to the ERAS group with P <0.001 (Table 5). In addition, the MAP in the non-ERAS group showed a significant elevation from 5 min intraoperative till 12 hours postoperative compared to the ERAS group with P <0.001 (Table 6).
Headache and dizziness were the two most frequent side effects reported among both groups, with no statistical difference.
| Intra-operative HR beat/min | Non-ERAS | ERAS | P-value |
Post- operative HR beat/min |
Non-ERAS | ERAS | P-value |
|---|---|---|---|---|---|---|---|
| N=29 | N=29 | N=29 | N=29 | ||||
| Basal | (88-95) 91.2±2.2 |
(87-96) 91±2.3 |
0.767 | 1 H | (83-95) # 88.8±3 |
(71-80) # 76±2.6 |
<0.001* |
| After induction | (86-92) # 88.8±1.8 |
(86-92) # 89.1±1.6 |
0.593 | 2 H | (84-91) # 87.2±2.1 |
(75-83) # 79.1±2.2 |
<0.001* |
| 5 min | (83-90) # 86.4±1.8 |
(76-83) # 79.8±1.7 |
<0.001* | 4 H | (81-88) # 84.7±1.9 |
(75-86) # 81.9±2.6 |
<0.001* |
| 10 min | (83-88) # 85.7±1.3 |
(67-74) # 69.8±1.7 |
<0.001* | 6H | (82-90) # 86.3±2.1 |
(78-86) # 82.7±2.1 |
<0.001* |
| 15 min | (82-89) # 84.8±1.8 |
(64-71) # 67±1.8 |
<0.001* | 8 H | (83-93) # 87.1±3.1 |
(79-87) # 81.9±2.2 |
<0.001* |
| 20 min | (82-88) # 85.3±1.4 |
(62-69) # 65.6±1.9 |
<0.001* | 10H | (81-93) # 85.4±3.1 |
(78-84) # 80.8±1.8 |
<0.001* |
| 30 min | (83-90) # 85.6±1.7 |
(63-69) # 65.6±1.5 |
<0.001* | 12 H | (80-87) # 83.9±1.8 |
(77-87) # 82.4±2.2 |
0.006* |
| 45 min | (84-91) # 87.8±1.5 |
(62-67) # 64.2±1.2 |
<0.001* | 18 H | (79-87) # 83.6±2.1 |
(81-87) # 83.9±1.4 |
0.513 |
| 60 min | (88-96) # 92.3±2 |
(65-71) # 68.4±1.4 |
<0.001* | 24 H | (81-90) # 86.3±2.6 |
(84-89) # 86.3±1.3 |
0.999 |
Independent Samples T-test and Paired Samples T-test were used for analysis.
#:Significant level at P-value < 0.05 in comparison with baseline data within each group.
*P is significant when at <0.05.
| Intraoperative MAP(mmHg) | Non-ERAS | ERAS | P-value | Postoperative MAP(mmHg) | Non-ERAS | ERAS | P-value |
|---|---|---|---|---|---|---|---|
| N=29 | N=29 | N=29 | N=29 | ||||
| Basal | (89-101) 94.3±3.4 |
(89-101) 93.8±3.5 |
0.574 | 1 H | (81-90) # 85.7±2.5 |
(69-78) # 73.8±2.3 |
<0.001* |
| After induction | (82-95) # 88.3±3.6 |
(84-96) # 88.8±3.5 |
0.605 | 2 H | (82-88) # 85.3±1.6 |
(72-79) # 75.3±2 |
<0.001* |
| 5 min | (79-91) # 85.5±3.1 |
(79-89) # 82.2±2.7 |
<0.001* | 4 H | (83-89) # 85.2±1.6 |
(75-82) # 77.8±1.8 |
<0.001* |
| 10 min | (79-89) # 82.7±2.7 |
(71-81) # 75±2.6 |
<0.001* | 6H | (81-87) # 83.4±1.6 |
(74-83) # 79.1±2.3 |
<0.001* |
| 15 min | (76-90) # 81.6±4.2 |
(66-76) # 70.6±2.6 |
<0.001* | 8 H | (80-88) # 84.3±1.9 |
(75-86) # 80.2±2.9 |
<0.001* |
| 20 min | (74-86) # 80.1±3.4 |
(64-71) # 67.4±2.2 |
<0.001* | 10H | (81-87) # 84.2±1.5 |
(78-86) # 82.6±2 |
<0.001* |
| 30 min | (77-88) # 81.7±3.2 |
(64-69) # 66.7±1.4 |
<0.001* | 12 H | (81-88) # 84.4±2.1 |
(79-86) # 81.8±1.7 |
<0.001* |
| 45 min | (76-89) # 82.3±3.2 |
(59-70) # 65.7±2.8 |
<0.001* | 18 H | (83-89) # 85.7±1.6 |
(80-89) # 84.9±2.1 |
0.108 |
| 60 min | (84-95) # 89±2.8 |
(65-74) # 70.2±2.2 |
<0.001* | 24 H | (83-93) # 88.9±2.7 |
(84-91) # 87.5±2 |
0.056 |
Independent Samples T-test and Paired Samples T-test were used for analysis.
#: Significant level at P-value < 0.05 in comparison with baseline data within each group.
*P is significant when at <0.05.
4. DISCUSSION
Currently, LSG is a popular bariatric operation. Anastomotic bariatric procedures are being rapidly replaced by this kind of minimally invasive surgery [24]. Like any other major abdominal surgery, the problems associated with LSG are not unique. Not only are technical issues well-documented in the literature, but perioperative problems are also addressed. A frequent issue following laparoscopic bariatric procedures is PONV. Early post-LSG hours are severely impacted by PONV, with a reported incidence of up to 90% [25]. Although researchers have worked tirelessly to lower the rate of PONV, it appears unlikely that PONV will ever completely disappear due to technological issues with LSG. A number of studies have been conducted to reduce PONV in the postoperative period in bariatric patients, with varying degrees of success. All of the research, however, is united by the same assertion: “PONV is inevitable” [26].
Fortunately, PONV has drastically decreased since ERAS was used in recent years. Still, numerous antiemetic medications are utilized globally to reduce PONV in order to address this issue [27].
Haloperidol, dexamethasone, and ondansetron have been selected because they each have distinct antiemetic action mechanisms and work well, whether taken separately or in combination, to prevent PONV. Patients in this study were given the same kind of antiemetic. In addition, each group's distribution of the variables Apfel, BMI, ASA physical status, weight, height, age, and gender was homogeneous. Therefore, the variations in peri- operative treatment protocols may be the cause of the discrepancies in PONV seen among the groups. In our study, 58 patients underwent elective LSG operation, 29 patients within ERAS protocol, and 29 patients under standard care protocol.
The ERAS group had a much lower incidence of PONV than the non-ERAS group in the first 36 hours after LSG, and the non-ERAS group had significantly greater PONV severity than the ERAS group. In addition, the first rescue antiemetic medication was taken later by the ERAS group than by the non-ERAS group; consequently, fewer patients in the ERAS group than in the non-ERAS group required rescue antiemetic. The ERAS group needed less nalbuphine than the non-ERAS group in terms of post- operative opioid intake. Compared to the non-ERAS group, the patients in the ERAS group were able to tolerate oral fluids more quickly. When it came to the assessment of pain in both groups, the non-ERAS group's VAS score was considerably higher than the ERAS group's. There were a few minor complications, but the two groups did not differ in any noticeable way.
Our results align with the findings of Benevides et al. [13], who divided their study into three groups based on the drug administered (Group O: ondansetron (8 mg); Group DO: ondansetron and dexamethasone (8 mg); and Group HDO: ondansetron, dexamethasone (8 mg), and haloperidol (2 mg). They observed the first 36 hours after LSG surgery, recorded the PONV, and discovered that the three antiemetic drug combinations combined decreased the incidence of vomiting by 63% as opposed to onda- nsetron alone itself, which is comparable to our PONV incidence in the non-ERAS group (51.7%). Benevides et al. [13] also recorded that, according to the quantity of antiemetic given as prophylaxis, there was a longer period of time in their trial before the first rescue antiemetic was administered. Moreover, less antiemetic was needed when haloperidol, dexamethasone, and ondansetron were taken together in their study.
Furthermore, a different trial conducted by Chu et al. [28] found that preventive haloperidol + dexamethasone reduced the incidence of PONV more than either medication alone did. The randomized, double-blind trial by Grecu et al. [29], which included 263 patients with a significant PONV risk, demonstrated that the combination of haloperidol and ondansetron prolonged the time to first rescue antiemetic more than the use of ondansetron alone.
Current guidelines of ERAS society recommendations advocate for a multimodal strategy that minimizes intra- and postoperative opioid use, avoids volatile anesthetics and fluid overload, and uses total intravenous anesthesia with propofol (TIVA); furthermore, opioid-sparing tech- niques, such as regional anesthesia and multimodal analgesia are advised in order to further lower the risk of PONV [16]. In our study, the ERAS group received TIVA with multimodal analgesics and TAP block to minimize intra- and postoperative opioid use and consequently minimize the incidence of PONV.
Consistent with our work, the RCT involving 119 patients with opioid-free TIVA was linked to a significantly decreased rate and severity of PONV compared to volatile anesthesia with opioids [20]. To further lower the incidence of PONV, ERAS, multimodal analgesia, and regional anesthesia techniques are advised as opioid-sparing measures [30]. Using short-acting medications and avoiding opioids as much as possible during the procedure is crucial to improve recovery [16].
Congruent with our attempts, the prevalence of PONV has decreased by 18%, based on randomized clinical trial meta-analysis using ERAS pain treatment pathways [31]. About 38% of patients using epidural analgesia and 78% using non-opioid analgesia, with 300 individuals receiving various colorectal surgeries participated in a multicenter clinical trial, in which the incidence of PONV was incredibly low (12%), especially when one considers that only 27% of research participants received antiemetic prophylaxis [32]. Another study, however, discovered that there was no statistically significant difference in the incidence of PONV or antiemetic usage between patients who underwent TIVA with remifentanil and propofol versus inhalational anesthesia with opioids [33]. Two observational studies found that 67–68% of patients with PONV required rescue antiemetics, with an average wait time of 136–142 minutes, among patients who were overweight and undergoing TIVA using propofol-remifentanil plus intravenous ondansetron, beta- methasone, or droperidol for antiemetic prophylaxis [34, 35]. The results of this study were different from our results as we used other techniques for postoperative pain relief like TAP block, with Lidocaine and Magnesium sulfate infusion with Paracetamol and Ketorolac, such as the study by Alimian and his colleague, who injected lidocaine 1 mg/kg/h IV or 2 mg/kg/h IV in the context of Opioid-free General Anesthesia (OFA), which similarly produced significantly decreased rates of PONV for each dose through 24 h [36]. It was discovered that using the ERAS protocol for bariatric surgery was successful in lowering peri-operative opioid intake and PONV incidence [37-39].
Opioid consumption in our study was decreased in the PACU and ward as a result of the combination of multimodal analgesics plus bilateral TAP block, which decreased the number of occurrences and the NRS score of> 4. This result was consistent with the findings of Ibrahim et al. [40], who used bilateral oblique subcostal transverse abdominis plane block guided by ultrasonography with iv fentanyl (1 μg/kg) and dexmedetomidine 0.1 μg/kg and ketamine (0.5 mg/kg) at induction, followed by dexmedetomidine 0.5 μg/Kg/h, ketamine 0.5 mg/kg/h, and lidocaine 1 mg/kg/h for the maintenance opioid-free group (n = 51). In contrast, patients in the multimodal analgesia group (n = 52) received only iv fentanyl (1 μg/kg) at induction. They confirmed that combining opioid-free anesthesia with local, regional anesthesia is a superior way to meet ERAS objectives. It minimizes the need for opioids over multimodal analgesia, enhances postoperative pain, and improves the quality of recovery early on. Additionally, Ma and his colleague [41] found a favorable association between the pain score and postoperative morphine consumption. They also found that merely implementing ERAS reduced the morphine equivalent dose by 41.8% (P < 0.001).
Using the validated QoR-15 questionnaire, we found that in our study, ERAS improved both the individual and overall quality of recovery ratings when compared to non-ERAS. This is in accordance with the findings of Ibrahim and his coworkers [40]. They found that the improvement in the pain component of the QoR-40 at 6 h in the OFA group was further corroborated by a lower mean NRS score at 6 h in the same group.
5. LIMITATIONS
The lack of ERAS compliance reporting, variable protocol features, study heterogeneity, and unclear stratification of morbidity categorization have reduced the strength of the findings of ERAS in bariatric surgery. Therefore, there are several restrictions in our investi- gation. First the long-term consequences of the ERAS process could not be assessed by our investigation. Second, in our center, we only prescribe elective LSG for patients with a BMI of ≥ 35 kg/m2. Due to this, we were only able to recruit members of this particular population, which resulted in a somewhat small sample size and might have limited the generalizability of our findings.
CONCLUSION
Despite the triple PONV prophylaxis of the standard care protocol to prevent PONV, our study found that, when the ERAS protocol was implemented, there was a significant reduction in the incidence and severity of PONV, as well as in the amount of opioids consumed postoperatively, the time it took to first administer a rescue antiemetic drug, the number of times it was administered, and improvement in the recovery quality.
AUTHORS’ CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| ERAS | = Enhanced Recovery After Surgery |
| LSG | = Laparoscopic Sleeve Gastrectomy |
| PONV | = Postoperative Nausea and Vomiting |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This clinical trial was approved by the Minia University ethical committee No. 820:6/2023 on July 1, 2023, and conducted in Minia University Hospital, Egypt before patient enrollment.
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analyzed during the current study are available from the corresponding author [H.S.M] upon reasonable request.
ACKNOWLEDGEMENTS
We are appreciate and immensely grateful to Prof Ibrahim Abbas Youssef Professor and founder of Anesthesiology, Intensive care and pain management Faculty of Medicine, El Minia University. Dr. Ibrahim contributed valuable input on study design and provided independent review and critique of the manuscript.


