All published articles of this journal are available on ScienceDirect.
Cross-cultural Adaption of the Indonesian Version of the Withdrawal Assessment Tool Version-1
Abstract
Background
Sedative and analgesic medications are commonly administered in the Pediatric Intensive Care Unit (PICU) but can cause complications, such as Iatrogenic Withdrawal Syndrome (IWS). The Withdrawal Assessment Tool Version-1 (WAT-1) questionnaire is a validated and reliable diagnostic tool for IWS, but it is not yet available in the Indonesian language.
Methods
This observational cross-sectional study was conducted in two phases at Ciptomangukusumo Hospital, Jakarta, Indonesia. The initial phase involved translating the WAT-1 and State Behavioral Scale (SBS) instrument into Indonesian. Subsequently, the validity and reliability of the Indonesian version of WAT-1 were tested on 30 patients who received sedative and analgesic medications for at least five consecutive days in the PICU. Evaluations were performed by two groups of nurses: PICU nurses and training nurses.
Results
The Indonesian version of WAT-1 was found to be valid and reliable. The validity showed correlations ranging from good to very strong (r = 0.490 to 0.836) among PICU nurses and strong correlations (r = 0.634 to 0.808) among trained nurses. The WAT-1 questionnaire demonstrated reliability with Cronbach's alpha values of 0.791 for PICU nurses and 0.785 for trained nurses. The Intraclass Correlation Coefficient (ICC) for WAT-1 indicated very good to excellent consistency in assessments, and the ICC for SBS indicated excellent consistency.
Conclusion
The Indonesian version of WAT-1 and SBS is a valid and reliable diagnostic tool for detecting Iatrogenic Withdrawal Syndrome in Children.
1. INTRODUCTION
Sedative and analgesic medications are frequently employed in the management of critically ill children in the Pediatric Intensive Care Units (PICUs), particularly for those undergoing mechanical ventilation. The primary goals of this therapeutic approach include enhancing patient comfort by alleviating pain or anxiety, improving synchronization between patient breathing and the ventilator, facilitating invasive procedures, and preventing the disconnection of medical devices [1, 2].
While these medications are beneficial, their prolonged use can lead to Iatrogenic Withdrawal Syndrome (IWS). Iatrogenic Withdrawal Syndrome is a clinical syndrome that emerges following the abrupt discontinuation or tapering of medication after extended use. Known risk factors for IWS include drug accumulation, duration of therapy, dose reduction strategies, patient age, and gender [3].
The diagnosis of IWS poses challenges due to symptoms overlapping with other intensive care conditions. To address this, several clinical scoring systems have been developed, including the validated and reliable Withdrawal Assessment Tool Version-1 (WAT-1), but it is not yet available in the Indonesian language.
The WAT-1 consists of four categorical assessments: reviewing nursing documentation from the previous 12 hours, a two-minute pre-stimulus observation, evaluation during progressive arousal stimulus, and post-stimulus observation [3]. During the two-minute observation period and one-minute post-stimulus, the WAT-1 utilizes the State Behavioral Scale (SBS) to assess the patient's level of consciousness. Consequently, the SBS has become an integral part of the WAT-1 questionnaire. Misinter- pretations may arise when implementing the English versions of the WAT-1 and SBS questionnaires since Indonesian is the primary language, and many members of the medical team are not fluent in English. Therefore, this study aims to translate and validate the WAT-1 (including the SBS) questionnaire into Indonesian to facilitate its use as a diagnostic tool in PICU settings.
2. METHODS
This observational cross-sectional study was conducted at CiptoMangunkusumo Hospital (RSCM), Jakarta, Indonesia (the top national referral hospital in Indonesia). The research was done in two phases: the initial phase involved translating both the WAT-1 questionnaire and SBS into Indonesian. In the second phase, we conducted validation and reliability testing for the Indonesian language version of WAT-1 and SBS instruments. The original creators of the WAT-1 and SBS have granted permission for the translation and validation of these instruments.
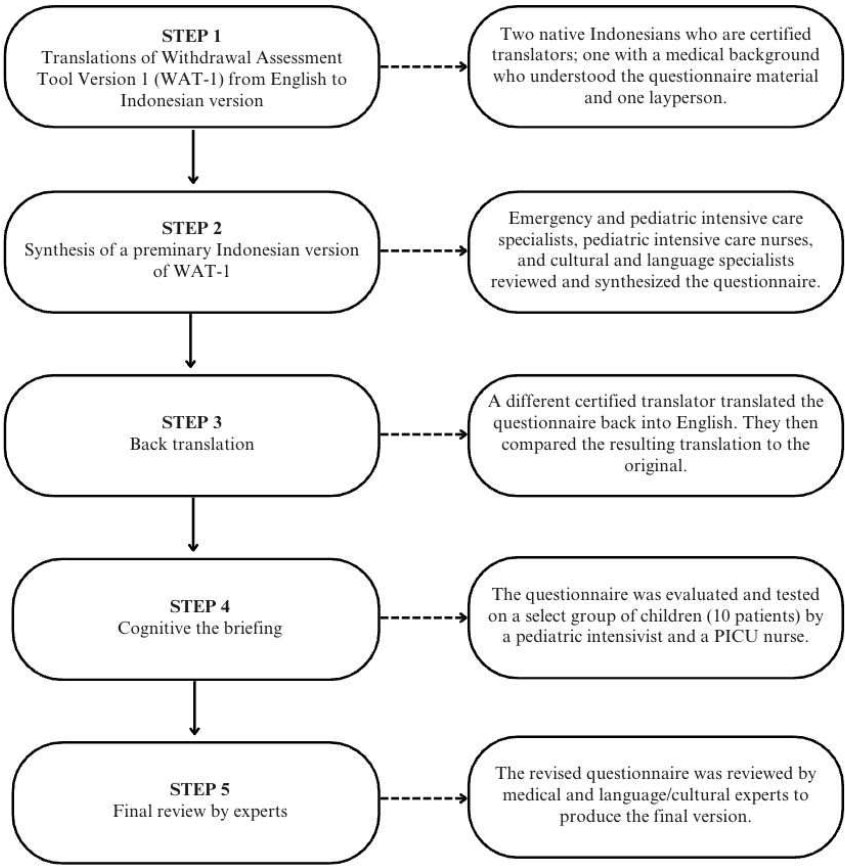
The initial phase of the study involved forward translation, synthesis of the translation and expert review, backward translation, cognitive debriefing, and final review (Fig. 1). The culmination of these steps resulted in the Indonesian version of WAT-1 and SBS instruments. The original English version of the WAT-1 and SBS instruments were translated into Indonesian (forward translation) by a certified translator. This translation was subsequently reviewed for accuracy by experts, including cultural and language specialists, emergency and pediatric intensive care specialists, and pediatric intensive care nurses (synthesis of the translation and expert review). The Indonesian text was then translated back into English (backward translation) by a different certified translator under the supervision of the original instrument creators. During the cognitive debriefing phase, the Indonesian versions of the WAT-1 and SBS instruments underwent evaluation and testing on a select group of children (10 patients) by a pediatric intensivist and a PICU nurse at CiptoMangunkusumo Hospital in Jakarta, Indonesia. A final expert review resulted in the approved Indonesian version of WAT-1 and SBS questionnaires.
In the second phase, we conducted validity and reliability testing for the final Indonesian version of the WAT-1 and SBS questionnaires. Testing involved 30 PICU patients who were selected consecutively. Inclusion criteria were patients aged one month to 18 years who had received sedative and analgesic therapy via infusion for more than five consecutive days without a dose reduction. Patients with muscle tone disorders, such as cerebral palsy, myasthenia gravis, and Guillain-Barré syndrome, were excluded. The assessments were carried out simultaneously by two nurses: a PICU nurse and a nurse trained in using the WAT-1 and SBS questionnaires in Indonesian. After a one-hour interval, both nurses reassessed the study subjects, with no communication permitted between them.
Data analysis was conducted using SPSS software version 29.0. We employed Pearson’s correlation between each domain score and the total score for validity testing. Correlation coefficient (r) ranged from 0 to 0.2, indicating poor correlation; 0.21 to 0.4, indicating low correlation; 0.41 to 0.6, indicating good correlation; 0.61 to 0.8, indicating strong correlation; and 0.81 to 1, indicating very strong correlation. Cronbach’s alpha and intraclass correlation coefficient (ICC) were utilized to assess inter-observer reliability. Cronbach’s alpha coefficient range from 0 to 0.20 was defined as unreliable, 0.21 to 0.40 as slightly reliable, 0.41 to 0.60 as moderately reliable, 0.61 to 0.80 as reliable, and 0.81 to 1 as highly reliable [4]. Internal consistency was measured using the intraclass correlation coefficient for each version. This included evaluating the consistency between assessments conducted by PICU nurses and those by nurses trained to use the WAT-1 and SBS questionnaires in the Indonesian language. We Also assessed absolute agreement between these two groups. ICC interpretation classifications were as follows: ICC less than 0.5 indicated poor consistency, 0.5 to 0.75 indicated good consistency, 0.75 to 0.9 indicated very good consistency, and greater than 0.9 indicated excellent consistency [4].
3. RESULTS
The initial stage of the research involved translation and cultural adaptation of the WAT-1 and SBS from English to Indonesian. This process included forward translation, expert review, backward translation, and cognitive debriefing. During the adaptation phase, we refined the terminology to suit cultural sensitivities; for example, the phrase “any vomiting/wretching/gagging” was translated to “muntah/tersedak” since “wretching” and “gagging” are synonymous in Indonesian. The study was conducted on 30 participants (Table 1), consisting of 60% females and 40% males. The sedative drugs most commonly used included midazolam in 8 patients, followed by dexmedetomidine in 4 patients, and 12 patients received both. Opioids were less frequently used, with 3 patients receiving fentanyl and 3 receiving morphine. The examinations were conducted by the pediatric ICU nurses and training nurses, with positive findings indicated by a WAT-1 score of 3 or greater observed in 26.6% of the patients.
Flux chart of study in the initial phase.
Table 1.
| Characteristic | Result | |
|---|---|---|
| Gender (n,%) | Male | 12 (40) |
| Female | 18 (60) | |
| Age in a month (median, IQR) | 18 (57) | |
| Diagnosis (n,%) | Pneumonia | 11 (36.7) |
| Acute respiratory distress syndrome | 6 (20) | |
| Septic or septic shock | 7 (23.3) | |
| Surgical | 4 (13.3) | |
| Airway problems | 1 (3.3) | |
| Heart failure in congenital heart disease | 1 (3.3) | |
| Drugs (n,%) | Midazolam | 8 (26.6) |
| Dexmedetomidine | 4 (13.3) | |
| Midazolam, dexmedetomidine | 12 (40) | |
| Midazolam, fentanyl | 1 (3.3) | |
| Dexmedetomidine, fentanyl | 2 (6.6) | |
| Dexmedetomidine, morphine | 2 (6.6) | |
| Midazolam, dexmedetomidine, morphine | 1 (3.3) | |
| WAT-1 scores (n,%) | Score ≥ 3 | 8 (26.6) |
| Score < 3 | 22 (73.3) | |
The validity of the PICU nurse report demonstrated a correlation coefficient range from 0.490 to 0.836, indicating a good to very strong correlation. The training nurse report showed a correlation coefficient range from 0.634 to 0.808, indicating a strong correlation. The results of this validation are presented in Table 2, illustrating the effectiveness of the Indonesia version of the WAT-1.
The reliability of the Indonesian version of the WAT-1 was assessed using Cronbach's alpha, which indicated that the instrument is reliable for both evaluative groups. The Cronbach's alpha values were similar across groups, with a value of 0.791 for the PICU nurse group and 0.785 for the group of nurses trained specifically in the use of WAT-1.
The consistency of assessments was evaluated using the Intraclass Correlation Coefficient (ICC). The result demonstrated excellent consistency in the PICU nurse group and good consistency in the trained nurse group. The Intraclass Correlation Coefficient (ICC) was also used to assess the absolute agreement between both groups, with the finding indicating very excellent agreement (Table 3).
The Withdrawal Assessment Tool Version-1 (WAT-1) includes an evaluation of the patient’s condition using the State Behavioral Scale (SBS). Reliability testing for this component was performed using the ICC. The results obtained are detailed in Table 4, demonstrating alignment with the established reliability standards for the tool.
| WAT-1 Indonesia Version | PICU Nurse | Training Nurse |
|---|---|---|
| Information from patient record, previous 12 hours | 0.490* | 0.634* |
| 2-minute pre-stimulus observation | 0.832* | 0.808* |
| 1-minute stimulus observation | 0.723* | 0.681* |
| Post-stimulus recovery | 0.836* | 0.788* |
| Parameters | ICC | 95% Confidence Interval | |
|---|---|---|---|
| Lower Bound | Upper Bound | ||
| Internal consistency of PICU nurse | 0.946 | 0.886 | 0.974 |
| Internal consistency of training nurse | 0.878 | 0.743 | 0.942 |
| Absolute agreement PICU and training nurse | 0.956 | 0.907 | 0.979 |
Table 4.
| Parameters | ICC | 95% Confidence Interval | |
|---|---|---|---|
| Lower Bound | Upper Bound | ||
| Internal consistency of PICU nurse | 0.981 | 0.960 | 0.991 |
| Internal consistency of training nurse | 0.967 | 0.930 | 0.985 |
| Absolute agreement PICU and training nurse | 0.874 | 0.713 | 0.942 |
4. DISCUSSION
The validity and reliability of research instruments are essential for ensuring accurate and consistent results [5]. The research findings indicate that all variables tested for validity are statistically significant within both the training nurse and PICU nurse groups. These results suggest that all healthcare professionals, particularly nurses, can effectively use the questionnaires. Previous validity assessments of the WAT-1 have demonstrated good psychometric performance and generalizability when assessing clinically significant withdrawal symptoms in both pediatric intensive care and general ward settings [6].
The Indonesian version of WAT-1 is a reliable instrument, evidenced by Cronbach's alpha values of 0.791 for the PICU nurse and 0.785 for the trained nurse groups, respectively. In this study, each patient underwent assessments conducted twice by two different nurses: a PICU nurse and a nurse trained in the WAT-1 application. Comparisons of the examination results between the two groups revealed excellent absolute agreement (r=0.956, 95% CI 0.907-0.979). These findings align with the previous research that reported an ICC value of 0.98 [7].
The State Behavioral Scale is a recommended tool for assessing levels of sedation, including agitation, which is a symptom of iatrogenic withdrawal syndrome. The Withdrawal Assessment Tool version 1 employs the SBS to evaluate calmness during a 2-minute observation period before any stimulus is applied. It also measures the time it takes for a patient to return to calmness, indicating an SBS value of zero or less after receiving a stimulus. Previous research on the reliability of the SBS reported an interclass correlation coefficient of 0.79 [8]. In this study, we also conducted a reliability test for the SBS and obtained an ICC of 0.874, indicating very good consistency.
The WAT-1 assessment instrument is a clinical examination tool that can be used to diagnose Iatrogenic Withdrawal Syndrome (IWS). However, it is not currently available in the Indonesian language. Given that these assessments are primarily conducted by nurses, many of whom are not proficient in English, translating and adapting the WAT-1 into Indonesian is considered essential.
The WAT-1 instrument consists of 11 clinical questions categorized into four groups. A score of 3 or higher (on a scale of 0-12) suggests a suspicion of IWS [3]. In our study, we observed that 8 out of 30 patients (26.6%) had a WAT-1 score of 3 or greater. Other studies utilizing the WAT-1 as a diagnostic tool for IWS have reported incidence rates with WAT-1 of 3 or greater at 47%, 64.9%, and 95% [9-11]. The incidence rate in our hospital is significantly lower compared to these figures, likely because the primary aim of this study was validation testing, with only four assessments conducted per patient. Ideally, monitoring for IWS using WAT-1 should be performed every 12 hours, starting from the day of weaning and continuing until 72 hours post-discontinuation of sedative and analgesic medication.
Furthermore, our hospital lacks a specific protocol for sedation, analgesia, and monitoring of IWS in patients, which means these examinations are not routinely conducted. Consequently, the actual incidence rate of IWS might be higher than what our study suggests. Literature indicates that various factors, including the type of drugs used, their dosages, and the duration of usage, influence the variation in incidence rates [9, 12, 13].
All patients with WAT-1 scores of 3 or greater were diagnosed with IWS and required gradual weaning from sedative-analgesic agents via continuous infusions. The withdrawal symptoms subsided concomitantly with the weaning process. Symptoms usually manifest between 8 and 48 hours after discontinuation and include autonomic dysregulation, gastrointestinal disturbances, central nervous system arousal, and motor abnormalities. Iatrogenic withdrawal symptoms were induced by sedative-analgesic agents, with opioids, such as fentanyl and morphine, and benzodiazepines, such as midazolam and lorazepam, being the most common. Other medications, including dexmedetomidine, propofol, and neuromuscular blocking agents, have also been reported as causative agents of IWS. In our study, the majority of patients diagnosed with IWS were administered with midazolam for more than five days [14].
The WAT-1 is recognized as an instrument with high sensitivity and specificity for detecting IWS in patients administered with benzodiazepine and opioid drugs, with values of 86% and 88%, respectively [15]. Currently, there is a trend in our hospital towards the increasing use of α2-adrenergic agonists, particularly dexmedetomidine. We continue to employ the WAT-1 for monitoring and diagnosis based on the recommendations from the Society of Critical Care Medicine Clinical Practice guidelines, pending the validation of assessment methods for IWS associated with α2-adrenergic agonists. Screening for IWS involves a combination of symptoms related to α2-adrenergic agonists, such as unexplained hypertension and tachycardia, along with assessments using validated tools for opioids or benzodiazepine [16].
Along with the diagnosis, prevention of IWS has become a crucial aspect of pediatric critical care management. Strategies proven to reduce the severity or delay the onset of IWS include gradual weaning of sedative analgesic agents, transitioning to long-acting medications, daily interruption, sequential rotation of sedative-analgesic agents, and the use of epidural/ intrathecal methods for analgesia. Despite this effort, IWS remains a significant challenge in the PICU setting [16, 17].
CONCLUSION
In conclusion, the Indonesian versions of WAT-1 and SBS questionnaires are valid and reliable diagnostic tools for diagnosing iatrogenic withdrawal syndrome in children.
AUTHORS’ CONTRIBUTION
N.W.P., F.P., and F.P.: Contributed to the study conception and design; E.G. and J.M.: Performed data collection. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| PICU | = Pediatric Intensive Care Unit |
| IWS | = Iatrogenic Withdrawal Syndrome |
| WAT-1 | = Withdrawal Assessment Tool Version 1 |
| SBS | = State Behavioral Scale |
| ICC | = The Intraclass Correlation Coefficient |
| ENT | = Ear-nose-throat |
| GABA | = Gamma-Aminobutyric Acid |
| CNS | = Central Nervous System |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study received ethical approval from the Ethics Committee of the Faculty of Medicine, University of Indonesia – Cipto Mangunkusumo Hospital, Indonesia (Ilmu Kesehatan Anak FKUI-RSCM), under Protocol Number: 23-04-0518.
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
AVAILABILITY OF DATA AND MATERIAL
The data of current study are available from corresponding author, [N.P.], on a reasonable request.
ACKNOWLEDGEMENTS
We would like to extend our appreciation to the Hospital Director CiptoMangunkusumo Hospital (Supriyanto); Director of Human Resources, Education, and Research CiptoMangunkusumo Hospital (Dwi Fatan Lilyana); Head of Child Health Department, CiptoMangunkusumo Hospital, Universitas Indonesia (Fatima Safira Alatas); Coordinator of Research, Child Health Department, CiptoMangunkusumo Hospital, Universitas Indonesia (Putri Maharani T. Marsubrin).


