All published articles of this journal are available on ScienceDirect.
Dural Puncture Epidural Anesthesia versus Traditional Spinal Anesthesia for Rigid Cystoscopy: A Randomized Controlled Trial
Abstract
Background
Neuraxial anesthesia is the gold standard for urological operations. Hence, this study examined whether dural puncture epidural (DPE) anesthesia provides better pain reduction than traditional spinal anesthesia (SP) during rigid cystoscopy.
Objectives
This study set out to evaluate if DPE anesthesia offers improvement in pain relief compared to traditional SP for rigid cystoscopy.
Methods
This randomized controlled trial included 76 adults of both genders undergoing elective rigid cystoscopy. The participants were randomly divided into two equal-sized groups. Group SP received 3 ml of hyperbaric bupivacaine (0.5%0 and 25 mcg of fentanyl (0.5 ml). Group DPE received a 15-ml mixture of bupivacaine (0.25%) and 50 mcg of fentanyl over 5 minutes.
Results
The time to first request rescue analgesia was delayed in group DPE compared to group SP.
The Group SP showed faster sensory block than the Group DPE. The Group DPE exhibited longer sensory and motor blocks than SP. Pain score, number of patients who required rescue analgesia, and total dose of morphine consumption in the first 24 hours were significantly lower in group DPE than in group SP. Mean arterial pressure (MAP) was significantly lower at 5min, 10min, and 15min in group SP than in group DPE.
Conclusion
DPE provides superior analgesia than SP as it offers prolonged duration sensory and motor block, better pain control, lower need for rescue analgesia, and better hemodynamic stability; however, SP has a rapid onset of sensory block.
1. INTRODUCTION
Neuraxial anesthesia is the gold standard for urological operations [1]. Assessing the lower urinary tract with cystoscopy is essential to urologic practice. Nevertheless, efforts have been made to reduce the associated pain of cystoscopy [1, 2].
Spinal anesthesia (SP) is known for its utilization in endoscopic urological surgery due to its ability to quickly identify symptoms resulting from transurethral resection of prostate (TURP) syndrome, bladder perforation, and overhydration [3]. The subarachnoid space is a potential local anesthetic (LA) injection site, which can alter hemodynamics and respiratory function [4, 5].
There are three stages in the dural puncture epidural (DPE): a variation on the epidural technique using a Tuohy needle to pinpoint the epidural space, inserting a spinal needle via a dural hole that has been created, and then placing the epidural catheter [6]. The dural hole facilitates the passage of epidurally injected drugs into the subarachnoid space, leading to a quicker start of pain relief and improved distribution throughout the sacral region [6-8].
As far as we know, this study represents the initial investigation to compare DPE anesthesia with traditional SP for rigid cystoscopy. Thus, the overarching goal of the study was to determine if DPE anesthesia improves pain relief and delays the time to first rescue compared to traditional SP for rigid cystoscopy.
2. METHODS
This prospective randomized, controlled, double-blind study was conducted on 76 participants, both sexes, above 18, with American Society of Anesthesiologists (ASA) physical activity I-III admitted for elective rigid cystoscopy. The study was conducted from October 2022 to March 2024 after the approval of the Ethical Committee of Tanta University Hospitals, Egypt (Approval code: 35621/8/22) and registration on ClinicalTrials.gov (ID: NCT06507397). The patient provided informed written consent. Patients with body mass index > 35 kg/m2, a history of drug misuse, communication issues, conditions that would prevent them from receiving neuraxial anesthesia, and a history of allergy to LA were excluded.
2.1. Randomization and Blinding
Participants were assigned to two groups of equal size through a random allocation process, using closed, sealed, opaque, serially numbered envelopes that were opened by the chief nurse (who was not included in the research or data gathering) at the morning of surgery, to determine the group of each patient. Group I received SP, and Group II received DPE. Both the participants and outcomes assessor were unaware of the group allocation.
All patients underwent history taking, clinical examination, and laboratory investigations. Each patient was instructed about a numeric rating scale (NRS) for postoperative pain. NRS (0 signifies “no pain” while 10 signifies “the utmost suffering conceivable”).
We injected 2% lidocaine into the skin and then inserted an 18 G or 16 G cannula to establish intravenous access. A balanced crystalloid solution (10 ml/kg) was preloaded over ten minutes before neuraxial blocks in all patients.
A senior anesthesiologist conducted all neuraxial procedures at the L4-5 interspace with the patient seated and following strict aseptic protocol.
2.2. Spinal Anesthesia Technique
A 25-gauge spinal needle was used to find the subarachnoid area in the gap between the L4-5 vertebrae. Following cerebrospinal fluid (CSF) aspiration, patients were given 3 ml of 0.5% hyperbaric bupivacaine and 25 mcg of fentanyl.
2.3. DPE Technique
The patient was given LA, and a midline route was used to implant an 18-gauge Tuohy needle at the L4-L5 vertebral interspace utilizing a loss-of-resistance to saline technique. The epidural needle was utilized to puncture the dura mater using a 25-G spinal needle, and the presence of free cerebrospinal fluid flow was confirmed. After removing the spinal needle, a multi-orifice epidural catheter was inserted 4 cm into the epidural space, with the cranial side facing upwards [7]. After negative CSF and blood aspiration, the 5 mL test dose of 2% lidocaine hydrochloride was given. If no unusual symptoms were noted after five minutes, a solution containing 0.25% plain bupivacaine (14 ml) and 50 mcg fentanyl (1 ml), a total of 15 ml was given.
Following this, patients in both groups were positioned on their backs and given 5 liters of oxygen per minute.
The start of sensory loss was determined by the time it took from injecting a bolus dosage to the first sign of sensory block at the T10 level. This was measured by puncturing a sterile needle with a blunt edge with a pin. Two minutes following the administration of the medicine, we assessed the sensory level, then every five minutes for thirty minutes, and finally every fifteen minutes until the surgery was over.
The Breen Modified Bromage Scale (BMBS) was employed to determine the time it took for the motor block to begin in the lower limbs, from the end of the drug injection until grade 1 was achieved. From 1 (no motor block) to 6 (full block), this scale indicates the severity of the motor block. The assessment was conducted initially at two-minute intervals and then every three minutes after drug injection until 30 minutes. After that, the assessment was performed every 15 minutes until the end of the surgery.
Heart rate (HR) measurements were taken at baseline, 5min, 10min, 15min, and at the end of surgery. NRS at 0, 2, 4, 8, 12, 18, and 24 h in both groups was recorded.
After surgery, patients were given a regular schedule of pain medications. Every patient was given 1 gram of paracetamol every 6 hours. After 30 minutes, if the pain persisted until the NRS was less than 4, a 3 mg bolus of morphine was administered as a rescue analgesic.
Furthermore, data were recorded on the number of patients who needed rescue analgesics within one day following the operation.
The occurrence of side effects, such as hypotension and bradycardia, was documented. Hypotension was defined when the systolic blood pressure (SBP) dropped by more than 15% from the initial SBP measurement upon the patient's arrival in the operating room or any reduction in pressure accompanied by disabling symptoms (such as dizziness, yawning, and nausea).
The primary outcome was the assessment of the onset of anesthesia. The secondary outcomes included motor block onset time, incidence of side effects, number of patients requiring rescue analgesia, and dose within 24 hours.
2.4. Sample Size Calculation
The computation of the sample size was conducted using G. power 3.1. The sample size was determined to be N ≥36 in each group, considering the following factors: the study had a 95% confidence limit and 80% power, and a group ratio of 1:1. Based on a pilot study on five cases in each group, the mean (± SD) of analgesia was 10.3 ± 3.4 min with SP and 11 ± 3.8 min with DPE block. Two additional cases were included in each group to overcome the dropout, resulting in 38 needed cases.
2.5. Statistical Analysis
SPSS v27 (IBM3, Armonk, NY, USA) was employed to perform the statistical analysis. The Shapiro-Wilks test and histograms were employed to determine if the data distribution was normal. Quantitative parametric data were presented using mean and standard deviation (SD), and then the unpaired student t-test was used for analysis. Quantitative non-parametric data were presented using the median and interquartile range (IQR) before being evaluated with the Mann Whitney U test. When necessary, we used the Chi-square test or Fisher's exact test to examine the data from the qualitative variables displayed using percentages and frequencies. It was deemed statistically significant if the two-tailed P-value was less than 0.05.
3. RESULTS
Ninety-four individuals were evaluated for their eligibility to participate in the study. Out of these, eleven patients did not fulfill the requirements, and seven chose not to participate. The remaining patients were randomly allocated into two groups, each with 38 patients. All assigned patients were statistically analyzed and followed up, as shown in Fig. (1).
CONSORT flowchart of the enrolled patients.
There were no significant variations in demographics and duration of operation between the two groups, as shown in Table 1.
The time until the initial request for rescue analgesia was significantly longer in group DPE than in group SP (P <0.001). Compared to group SP, group DPE had a significantly lower number of patients requiring rescue analgesia and a lower total dose of morphine consumed within the first 24 hours (P <0.05). The DPE and SP groups showed no statistically significant changes in the NRS assessments taken at 2, 4, 18, and 24 hours. Nevertheless, the observed values exhibited a notable decrease at both the 8- and 12-hour marks in the group DPE than the group SP (P <0.05). Hypotension, bradycardia, and headache incidence were not significantly different between the two groups, as shown in Table 2.
The group DPE exhibited a significant delay in the onset of sensory block compared to the group SP (P value=0.008). No notable disparity was observed in the motor block onset when comparing the two groups. Group DPE exhibited significantly prolonged durations of sensory and motor blockages compared to group SP (P <0.05), as shown in Table 3.
HR measurements at baseline, 5min, 10min, 15min, and at the end of surgery were insignificantly different between groups. Mean arterial blood pressure (MAP) measurements were insignificantly different at baseline and the end of surgery and were significantly lower at 5min, 10min, and 15min in group SP than in group DPE (P <0.05), as shown in Fig. ( 2).
|
Group SP (n=38) |
Group DPE (n=38) |
P-value | ||
|---|---|---|---|---|
| Age (years) | 56.89 ± 9.53 | 59.84 ± 9.46 | 0.180 | |
| Sex | Male | 28 (73.68%) | 32 (84.21%) | 0.260 |
| Female | 10 (26.32%) | 6 (15.79%) | ||
| Weight (kg) | 81.13 ± 7.78 | 82 ± 10.51 | 0.683 | |
| Height (cm) | 170.24 ± 6.44 | 169.08 ± 7.65 | 0.477 | |
| BMI (kg/m2) | 28.13 ± 3.54 | 28.8 ± 4.05 | 0.446 | |
| ASA physical status | I | 9 (23.68%) | 8 (21.05%) | 0.716 |
| II | 13 (34.21%) | 16 (42.11%) | ||
| III | 16 (42.11%) | 13 (34.21%) | ||
| Duration of surgery (min) | 29.21 ± 7.31 | 30.26 ± 7.44 | 0.536 | |
|
Group SP (n=38) |
Group DPE (n=38) |
P-value | |||
|---|---|---|---|---|---|
| NRS | 0 h | 0 (0 - 0) | 0 (0 - 0) | 0.155 | |
| 2 h | 0 (0 - 0) | 0 (0 - 0) | 0.079 | ||
| 4 h | 1 (0 - 1) | 1 (0 - 1) | 0.364 | ||
| 8 h | 2 (1 - 3) | 1 (1 - 2) | 0.006* | ||
| 12 h | 2 (2 - 4.75) | 2 (1.25 - 2) | 0.031* | ||
| 18 h | 2 (2 - 4) | 2 (2 - 2.75) | 0.072 | ||
| 24 h | 3 (2 - 4) | 3 (2 - 3) | 0.211 | ||
| Number of patients required rescue analgesia | 25 (65.79%) | 16 (42.11%) | 0.038* | ||
| Time to first request of rescue analgesia (h) | 7.92 ± 2.2 | 11.69 ± 2.91 | <0.001* | ||
| Total dose of morphine consumption in the first 24 hours (mg) | 3.9 ± 2.6 | 1.7 ± 2.2 | <0.001* | ||
| Complications | Hypotension | 7 (18.42%) | 5 (13.16%) | 0.754 | |
| Bradycardia | 4 (10.53%) | 3 (7.89%) | 1 | ||
| Headache | 3 (7.89%) | 5 (13.16%) | 0.708 | ||
|
Group SP (n=38) |
Group DPE (n=38) |
P value | |
|---|---|---|---|
| Onset of sensory block (min) | 9.3 ± 3.9 | 11.5 ± 3.1 | 0.008* |
| Onset of motor block (min) | 13.5 ± 4.3 | 14.8 ± 3.2 | 0.118 |
| Duration of sensory block (min) | 490 ± 120.2 | 560.8 ± 139.8 | 0.021* |
| Duration of motor block (min) | 450.8 ± 120.6 | 582.1 ± 147.9 | <0.001* |
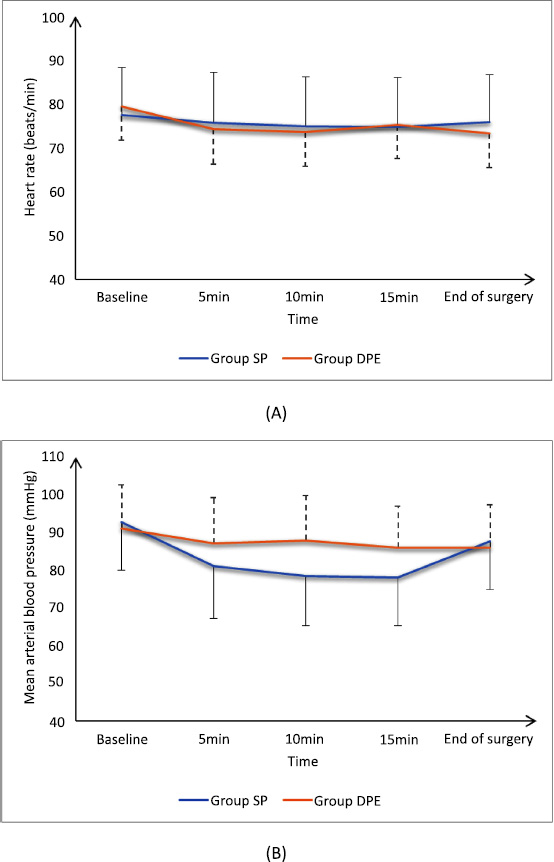
(A) Heart rate and (B) mean arterial blood pressure of the studied groups.
4. DISCUSSION
Bupivacaine is a long-acting local anesthetic that provides effective pain relief by blocking nerve transmission in the epidural space. The concentration of 0.25% is often used for procedures, such as labor epidurals or postoperative pain management, because it strikes a balance between providing adequate analgesia while minimizing motor blocking and allowing for better mobility [9]. A volume of 14 mL is typically sufficient to cover the relevant dermatomes required for effective anesthesia without excessive diffusion that could lead to unwanted side effects.
Based on our research, this is the first study that compares the effectiveness of DPE anesthesia vs regular SP anesthesia for rigid cystoscopy.
In this research, the time it took for the first request for rescue analgesia was longer in the DPE group compared to the SP group. The pain scores, number of patients requiring urgent rescue analgesia, and total dosage of morphine utilized during the first 24 hours were all considerably lower in the DPE group compared to the SP group. Compared to group SP, group DPE experienced a much later sensory block. The sensory and motor blocks of Group DPE lasted much longer than those of Group SP. Group DPE had significantly higher MAP values at 5, 10, and 15 min than group SP.
The DPE method was found by Wang et al. [10] to be more effective than the epidural in delivering anesthesia for a variety of reasons, including a faster start to surgical anesthesia, better diffusion of sensory block in the sacral and cranial regions, and a stronger motor block. Importantly, these benefits were achieved without increasing the occurrence of adverse effects in mothers undergoing repeat cesarean delivery.
Based upon our investigations, DPE was preferred over traditional SP because DPE enables a prolonged and regulated release of LA into the epidural space, thereby extending the analgesic effect compared to the shorter duration of SP [11]. With DPE, the anesthesia could be spread more slowly and more carefully. This lowers the risk of high spinal block, which may occur with standard SP [12, 13]. Thus, the prolonged analgesic effect decreases the necessity for supplementary analgesics during the postoperative period.
Research by Lin et al. [14] showed that using the DPE approach with 25-G spinal needles led to quicker pain relief onset, wider sacral coverage, increased sacrum spread, and reduced need for further epidural medication. Rao et al. [15] demonstrated that the epidural group had a longer delay before the sensory block occurred than the group that administered the DPE. The group that received an epidural was more likely to need additional top-ups than the group that received DPE.
In contrast, Sharawi et al. [16] stated that onsets of sensory blocks were significantly delayed in the epidural group than in group DPE.
Yin et al. [17] included 10 trials with 1,099 patients in their systematic review and meta-analysis. The study revealed that the utilization of the DPE approach during labor led to a higher percentage of patients reporting a pain score of 3/10 or lower at 10 and 20 min after receiving labor analgesia. DPE analgesia did not exhibit any negative consequences.
Chau et al. [7] reported that the group DPE had a decreased sacral block-sparing occurrence and asymmetric block compared to the epidural group. Nevertheless, the time it took for the analgesic effects to kick in was not significantly different. Furthermore, the group receiving DPE exhibited a reduced need for epidural top-ups in comparison to the group receiving regular epidurals.
When performing an epidural injection, some volume of the injected local anesthetic (like bupivacaine) and adjuncts (like fentanyl) can indeed migrate into the intrathecal space through the dural hole. Several factors can influence this movement, though the exact volumes can vary and are somewhat speculative. While it is challenging to predict exact migration volumes into the intrathecal space, some studies suggest that, following epidural injection, a certain percentage of the total volume may enter the intrathecal space. Common speculation might suggest that a small but clinically relevant portion of the total volume may migrate intrathecally, often considered less than 10% of the total dose [18].
In addition, the study had some limitations, such as a small sample size, the fact that it was conducted at just one center, and the short duration of patient follow-up. Further studies comparing other techniques are recommended.
CONCLUSION
DPE provides superior analgesia than SP as it offers a prolonged sensory and motor block duration, better pain control, lower need for rescue analgesia, and better hemodynamic stability; however, SP has a rapid onset of sensory block.
AUTHORS’ CONTRIBUTION
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| DPE | = Dural Puncture Epidural |
| SP | = Spinal Anesthesia |
| MAP | = Mean Arterial Pressure |
| TURP | = Transurethral Resection of Prostate |
| LA | = Local Anesthetic |
| CSF | = Cerebrospinal Fluid |
| BMBS | = The Breen Modified Bromage Scale |
| HR | = Heart Rate |
| SBP | = Systolic Blood Pressure |
| ASA | = American Society of Anesthesiologists |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was conducted from October 2022 to March 2024 after the approval of the Ethical Committee of Tanta University Hospitals, Egypt (Approval code: 35621/8/22).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
ACKNOWLEDGEMENTS
Declared none.


