All published articles of this journal are available on ScienceDirect.
Evaluating the Effect of Intraoperative Dextrose 10% Administration on Reducing Postoperative Nausea and Vomiting after Laparoscopic Surgery
Abstract
Background:
Although PONV is usually self-limiting or is treated without sequelae, the incidence of PONV could be as high as 70% to 80% in high-risk populations such as female sex, obese patients, age younger than 40 years, nonsmoker patients, history of PONV or motion sickness.
Objectives:
The study aimed to investigate whether dextrose 10% decreases the incidence of postoperative nausea and vomiting in female patients undergoing laparoscopic cholecystectomy
Materials and Methods:
This prospective, double-blind randomized placebo-controlled study comprised 130 ASA physical status I and II nonsmoker female patients, 20-40 years of age, scheduled for laparoscopic cholecystectomy at Ain Sham University – Assembled operating theater from August 2018 to October 2018.
Patients were arbitrarily divided into two study groups of 65 patients each. Group LR received lactated Ringer’s solution and group D received 10% dextrose. The primary objective of this study was to compare the incidence of PONV in the study treatment groups. The secondary outcomes included measurement of antiemetic medication consumption as well as blood glucose changes between groups.
Results:
50 from a total of 65 participants (76.9%) in Lactated Ringer (LR) group experienced nausea. On the other hand, 30 participants only (46.2%) in dextrose (D) group were nauseated. This dissimilarity was statistically highly significant (P= 0.0003).
Conclusion:
In this study, dextrose 10% administration resulted in improved postoperative emesis management as explained by the lower incidence of nausea and rescue antiemetic consumption.
1. INTRODUCTION
The term PONV is commonly described as nausea and/or vomiting or retching in the Post Anesthesia Care Unit (PACU) and in the first 24 postoperative hours. The revealed rate of PONV is assessed to be higher than 30% without prophylactic antiemetic medication.
The incidence of PONV could exceed 70% to 80% in high-risk populations, for example females, obese patients, age younger than 40 years, nonsmoker patients, previous history of PONV or motion sickness. Other risk factors are the lengthy duration of surgery, general anesthesia, use of inhalational anesthetics and nitrous oxide, opioids and laparoscopic procedures [1, 2].
Despite being self-limiting or treated without sequelae, PONV may result in considerable post-operative patient discomfort and dissatisfaction, it increases healthcare costs by increasing patients' stay in the PACU and hospital. Moreover, PONV can result in wound dehiscence, delayed oral intake, pulmonary aspiration, dehydration, electrolyte imbalance, acid-base disturbance increased intracranial pressure and pneumothorax. Hence, identifying effective strategies for PONV prophylaxis is vital, economical, improves medical outcomes and enhances patient safety and satisfaction [3-5].
Different pharmacological and non-pharmacological strategies have been tested for the prevention and treatment of PONV. Many antiemetic medications are available nowadays, antihistamines, metoclopramide, droperidol, and dexamethasone have been used; however, they have many unsatisfactory adverse effects, and in general, the most functional prophylactic approach has not been identified yet [5-8].
Two recently proposed strategies are perioperative fluid therapy and carbohydrate (dextrose) loading [9-13]. Many studies have reported the use of perioperative Intravenous (IV) dextrose therapy to decrease the occurrence and severity of PONV. The exact mechanism has not been determined yet, but it has been hypothesized that gastric mucosal hypoperfusion resulting from hypovolemia which may occur due to prolonged preoperative fasting, maybe a contributing factor leading to PONV. Since intravenous fluid loading can reduce hypovolemia and subsequently hypoperfusion, many studies were designed to evaluate the impact of dextrose-rich fluid administration on the incidence and severity of PONV through a mechanism probably involving hyperglycemia [14]. However, data available from these different clinical trials are limited and show conflicting results [15].
We, therefore, designed a double-blind, randomized, controlled trial to assess the ability of intraoperative infusion of dextrose 10% to prevent PONV and reduce postoperative use of antiemetic medication. Furthermore, to the best of our knowledge, no previous study in the literature has examined the efficiency of IV dextrose 10% administration on the rate and severity of PONV and the need for rescue antiemetic therapy in this high-risk patient group: female, nonsmoking, undergoing laparoscopic procedures.
2. MATERIALS AND METHODS
2.1. Selection of Patients and Randomization
This prospective, double-blind randomized placebo-controlled trial was approved by the Institutional Ethical Committee. Written informed consent was obtained from ASA physical status I and II nonsmoker female patients, 20-40 years of age, scheduled for laparoscopic cholecystectomy at Ain Sham University – Assembled operating theater.
The work was approved by the Research Ethics Committee of ASUHs (FMASU R 43/ 2018) and in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments in humans.
The study was prospectively registered with Pan African Clinical Trial Registry (PACTR) with Registration Number PACTR201808847015879 and conducted in the period from August 2018 to October 2018.
Patients were excluded for morbid obesity, severe hypertension, congestive heart failure, coagulopathy, significant hepatic or renal disease, diabetes mellitus, and abnormal blood glucose on the morning of surgery, or withdrawal of consent. Patients were also excluded from analysis for severe intraoperative hypotension requiring large volume intravascular fluid treatment or patients whose operations were prolonged (more than 2 h)
Patients with a previous history of PONV or currently receiving steroids or antiemetics, and those who were pregnant, or menstruating were also excluded from the study.
Patients who fulfilled the inclusion criteria were randomly allocated into two equally sized Groups RL and D (each group, n = 65) by a nurse anesthetist who was blinded to the study groups using a sealed-envelope technique and computer-generated random numbers. Patients' demographic data were also collected at this time. All patients fasted 8 h before surgery.
All patients in this study were subjected to a detailed pre-anesthetic evaluation. All basic investigations according to the hospital protocol (e.g. fasting blood sugar, serum hemoglobin, coagulation profile, liver function tests, kidney function tests, HbA1c, chest X-rays and Electrocardiogram (ECG)) were checked. Patients with HbA1c levels ≥ 6.5% (denoting undiagnosed Diabetes Mellitus) were excluded.
2.2. Anesthetic Technique
On the arrival to operation room; automatic blood pressure measurements, five-lead ECG monitor, and finger pulse oximetry were applied. An intravenous infusion of ringer was started, followed by intravenous metoclopramide 10 mg as an antiemetic.
The patients were arbitrarily divided into two study groups of 65 patients each as per a computer-generated code: lactated Ringer’s solution (group RL) or 10% dextrose (group D). The study fluid was delivered in opaque bags labeled “study fluid,” and infusion controlled by infusion pumps at 125 mL/h IV for 2 hours (250 mL) starting with the induction of Anesthesia. This volume was chosen to provide small glucose supplements.
After proper assessment of the airway and anticipation of the difficult airway, pre-oxygenation with 100% O2 on 8 L/min for 3 min via face mask was started. As for pre-medication, all patients received IV midazolam (0.02 mg/kg). Induction of anesthesia was achieved with propofol 2 mg/kg IV and fentanyl 2 μg/kg IV. Atracurium besylate 0.5 mg/kg IV was given to facilitate tracheal intubation, and anesthesia was maintained with 1 to 1.5% isoflurane. After orotracheal intubation, mechanical ventilation was started. For both groups, controlled mechanical ventilation was achieved by tidal volumes of 8-10 mL/Kg to avoid barotraumas and frequency of ventilation of up to 12-14 breaths/min to maintain normocapnia: end-tidal pressure CO2 (ET CO2) at the level of 35 ± 5 mm Hg and Positive End Expiratory Pressure (PEEP) of 5-10 cm H2O.
Patients were then positioned. During the procedure, pneumoperitoneum was established for maintaining the Intra-Abdominal Pressure (IAP) lower than 14 mm Hg.
Supplemental boluses of Atracurium besylate 0.1 mg/kg IV were administered every 20 minutes to maintain muscle relaxation during surgery.
Anesthesia was maintained with isoflurane 1-1.5% to maintain the HR and MAP within 20% of pre-induction values and/or Heart Rate (HR) < 85 beats/ min during surgical stimulation.
At the end of the surgery, each patient was extubated upon meeting the extubation criteria. All subjects were transferred to the PACU, where they were monitored for an additional 4 hours and got nasal O2 supplementation.
Hemodynamic parameters as HR and MAP were documented preceding premedication, before induction and after intubation, trailed by each 5 min for 30 min, from that point each 15 min till surgical procedure completes and after extubation in all patients.
Intraoperatively, any increase or decrease of HR, hypotension or hypertension was managed as required. For example, MAP rise of more than 20% above baseline was treated by administering a 0.5 μg/kg IV bolus of fentanyl and raising the end-tidal isoflurane concentration to 2%. MAP drop of more than 20% below baseline was dealt with at first with a decrease of the end-tidal isoflurane concentration to 1% and fast intravenous fluid bolus (250 ml crystalloids). If still hypotensive, 6 mg ephedrine was given intravenously with subsequent exclusion from the study.
Additionally, total intraoperative narcotic consumption was recorded.
The patients were transferred to the post-anesthesia care unit and were put under observation. Blood samples were analyzed by glucometer (Abbott Optium Xceed) for blood sugar level preoperatively, then every hour till the end of the surgery, and 4 h after surgery at the time of PACU discharge.
Severity of PONV was assessed using a 4-point Verbal Descriptive Scale (VDS) which consists of score 0 = no PONV: no complaint of nausea or vomiting; score 1 = mild PONV: patient complains from nausea but refuses antiemetic treatment; score 2= moderate PONV: patient complains from nausea and need antiemetic treatment; score 3 = severe PONV: patient complains from nausea with episode of vomiting requiring antiemetic treatment. PONV scores were obtained every half hour until discharge from the PACU. Rescue antiemetic ondansetron 4 mg IV was given when VDS scores were 2 or more. Medications were given only after excluding other causes of PONV such as hypovolemia, hypotension, hypoxia.
The total number of doses of antiemetics used and episodes of nausea and/or vomiting were obtained every half hour from the time of entry to the PACU until release.
The primary objective of this study was to compare the incidence of PONV (Yes/No) in the study treatment groups.
The secondary efficacy measures included frequency of vomiting episodes, measurement of PONV severity scores, need for rescue antiemetic and total antiemetic medication consumption. The antiemetic medication consumption was defined as total antiemetic medication doses consumed by each patient for the entire PACU stay as well as of blood glucose changes between groups.
2.3. Sample Size Determination
A sample of 130 patients (65 patients per each group) was calculated using power and sample size calculation program version 3. Using incidence of PONV in study group = 45.7% and in control group = 22.8% power of study 80% and α error = 0.05.
2.4. Statistical Analysis
Data were analysed using Statistical Package for Social Science (SPSS) version 21.0. Chicago, Illinois, USA. Quantitative data were expressed as mean ± standard deviation. Qualitative data were expressed as count. The independent-samples t-test was used to compare means in the two groups. Skewed numerical data are presented as median (range) and the independent samples-median test was used to compare between medians in both groups. Chi-square test was used to compare proportions between two qualitative parameters. P < 0.05 was considered significant and P < 0.01 was considered highly significant.
3. RESULTS
130 patients were enrolled and completed the study. Baseline demographic data of participants including age, ASA class, BMI and Hb A1c were comparable between the two groups. Duration of surgery and anesthesia, total I.V fluids received in the O.R, total intraoperative opioid consumption and estimated intraoperative blood loss were also statistically non-significant (Table 1).
50 of 65 participants (76.9%) in Lactated Ringer (LR) group experienced nausea versus 30 of 65 participants (46.2%) in dextrose (D) group, this dissimilarity was statistically highly significant (P = 0.0003) regarding incidence of vomiting, although lower in D group was statistically not significant between the two groups (Table 2).
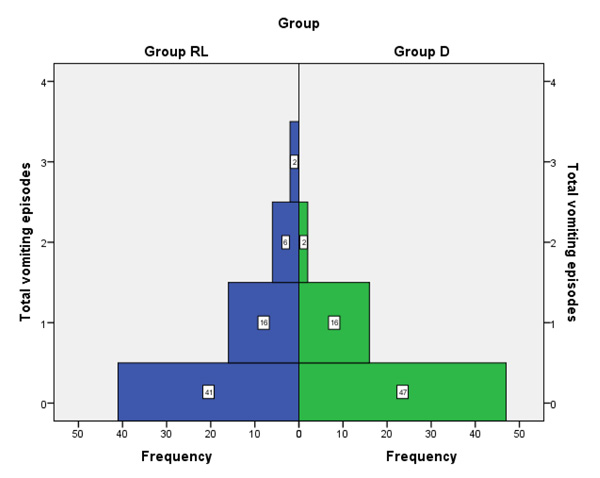
Fig. (1) shows the frequencies of vomiting episodes in both groups, 24 participants in LR group experienced one or more vomiting episodes, while only 18 participants in D group experienced one or more vomiting episodes.
PONV VDS (Verbal Descriptive Scale) were recorded postoperatively on arrival to the PACU (T0) and every 30 minutes thereafter for the first 4 hours postoperatively. In general, VDS scores were lower in D group rather than LR group irrespective of time point measured. The highest difference of scores were at 3 hours postoperatively (P = 0.006), and the overall mean VDS score during the first 4 hours postoperative was significantly lower in D group compared to LR group (P = 0.009) (Table 3).
48 of 65 participants (73.8%) in the LR group versus 26 of 65 participants (40%) in D group needed rescue antiemetic postoperatively, this variation was statistically significant. In addition, the total dose of antiemetic used in D group was significantly lower than that used in the LR group (Table 4).
| Variables |
Group LR (n=65) |
Group D (n=65) |
P value |
|---|---|---|---|
| Age (years) | 30.87 ± 5.42 | 31.40 ± 5.56 | 0.708# |
| BMI | 26.62 ± 1.58 | 27.55 ± 1.45 | 0.120# |
| ASA (I/II) | 36/29 | 39/26 | 0.605° |
| HbA1c | 4.43 ± 0.26 | 4.68 ± 0.46 | 0.111# |
| Duration of surgery (min) | 86.60 ± 3.88 | 85.70 ± 3.46 | 0.347# |
| Duration of anesthesia (min) | 99.10 ± 3.69 | 97.50 ± 3.57 | 0.094# |
| Intraoperative fluids (ml/kg/hr) |
1.69 ± 0.01 | 1.67 ± 0.07 | 0.098# |
| Intraoperative opioids (ug) | 221.05 ± 10.98 | 224.70 ± 10.52 | 0.063# |
| Intraoperative blood loss (ml) | 51.63 ± 4.04 | 49.83 ± 4.02 | 0.089# |
# Measured by independent t-test
°Measured by Chi-square test
All P values are nonsignificant (> 0.05)
| Variable |
Group LR (n=65) |
Group D (n=65) |
P value |
|---|---|---|---|
| Patients with nausea [n, %] | 50(76.9) | 30(46.2) | 0.0003° |
| Patients with vomiting [n, %] | 24(36.9) | 18(27.7) | 0.261 |
P-value Measured by Chi-square test
°significant p-value.
| VDS at Different Time Points |
Group LR (n = 65) |
Group D (n = 65) |
P value |
|---|---|---|---|
| VDS score T0 | 1(0-3) | 0(0-3) | 0.06 |
| VDS score T30 | 1(0-3) | 0(0-3) | 0.17 |
| VDS score T60 | 1(0-3) | 0(0-3) | 0.139 |
| VDS score T90 | 1(0-3) | 0(0-1) | 0.303 |
| VDS score T120 | 1(0-3) | 0(0-1) | 0.334 |
| VDS score T150 | 0(0-1) | 0(0-1) | 0.998 |
| VDS score T180 | 0(0-3) | 0(0-2) | 0.006* |
| VDS score T210 | 0(0-1) | 0(0-1) | 0.09 |
| VDS score T240 | 0(0-1) | 0(0-1) | 0.12 |
| Overall mean VDS score | 0.65±0.42 | 0.45±0.52 | 0.009* |
| Variables |
Group LR (n = 65) |
Group D (n = 65) |
P value |
|---|---|---|---|
| Patients received Rescue antiemetic [n, %] | 48(73.8%) | 26(40%) | 0.001° |
| Total dose of antiemetic used [mg] |
3.73 ± 2.76 | 2.00 ± 2.28 | 0.011# |
# Measured by independent t-test
°Measured by Chi-square test
Table 5 shows perioperative changes in blood glucose levels between the two groups. Participants in D group had higher blood glucose levels after the start of IV fluid infusion compared to those in LR group, though this difference was statistically not significant and all blood glucose values in all participants were within the normal range.
| Glucose Level(mg/dl) |
Group LR (n = 65) |
Group D (n = 65) |
P value |
|---|---|---|---|
| Preoperative | 100.8 ± 4.28 | 100.1 ± 4.14 | 0.523 |
| 60 min in the PACU | 110.03 ± 4.22 | 122.4 ± 4.34 | 0.145 |
| 120 min in the PACU | 116.77 ± 4.5 | 120.83 ± 5.31 | 0.103 |
| At time of discharge from PACU | 107.33 ± 4.04 | 108.23 ± 4.17 | 0.304 |
P values are measured by independent t-test. All p values are non-significant
4. DISCUSSION
Laparoscopic cholecystectomy is considered now a better substitution for traditional open cholecystectomy in patients suffering from cholecystitis; Unfortunately, Laparoscopic surgeries proved to show excessive episodes of PONV [16] with an incidence of approximately 30% [3, 4].
Although many different antiemetic drugs in many studies have been evaluated rather alone or with combination trying to prevent PONV after LC, unsatisfactory results occurred. Also, these drugs have many side effects including hypotension, hallucination, excessive sedation, dysphoria, and dry mouth [17].
Although no clear evidence regarding the efficacy of perioperative fluid therapy and glucose administration on PONV is proved, oral and IV carbohydrate-rich liquid has widely been used for the treatment of PONV with good results [13, 18]. However, some other studies did not confirm this result [19, 20].
The major finding of our study was that patients receiving intraoperative IV dextrose (group D) had significantly lower incidence and severity of nausea and vomiting and less frequently needed antiemetic medication postoperatively in comparison to the control group (Group RL).
These results agreed with the results of the study done by Atashkhoei et al. (2018) in which intraoperative dextrose 5% was used in patients undergoing Diagnostic Gynecologic Laparoscopy and found that there is significant decrease in the incidence and severity of PONV as well as delay in the first time to request for antiemetic after surgery and reduced total dose of antiemetic drugs used [21].
Consistent with our findings, Firouzian et al. concluded that the patients underwent LC and was given Dextrose 5%, 30 minutes pre-operatively had a significant decrease in the incidence and severity of PONV. They also less frequently needed antiemetic medication postoperatively in comparison to the control group [15].
Also, the study conducted by Dabu-Bondoc et al. showed a positive effect of giving glucose-containing IV fluids in the post-anesthesia period on the need for subsequent antiemetic use and length of PACU stay in healthy females undergoing outpatient gynecologic surgery [22]. They reported reduced doses of rescue antiemetic compared with the control group but was associated with alike nausea scores.
Irkal et al. made a study on patients undergoing tympanoplasty under general anesthesia. Although they found that postoperative nausea and vomiting scores were nearly the same between both groups and were not statistically significant, postoperative infusion of dextrose 5% intravenous improved postoperative emesis management [23].
These results matched with the results of Sada et al. who administrate pre-operative oral carbohydrates in patients who had undergone open cholecystectomy and reported a significant improvement of post-operative nausea as compared to those undergoing colorectal operations [18].
The idea of giving Glucose as anti-emetic is thought because of its high osmotic pressure that reduces muscle contractions in the gastrointestinal tract [22]. It also decreases gastric acid secretion that results in decrease Gastric muscle contraction [8] by inhibiting the vagal cholinergic pathways [24].
Another important role for IV dextrose containing fluids for improving PONV is to counteract tissue hypoperfusion which can be caused by Gastric mucosal hypoperfusion occurring due to hypovolemia after prolonged fasting. On the other hand, mucosal hypoperfusion proved to be caused by general anesthesia, increased intra-abdominal pressure due to pneumoperitoneum during laparoscopy, and surgical stimulation even without any decrease in blood pressure. So, intravenous administration of glucose-containing fluid can decrease this hypovolemia and hypoperfusion [25].
Some other studies have indicated that the occurrence of PONV and other postoperative complications depends on the postoperative insulin-resistance; a result of perioperative trauma. Therefore, peri-operative glucose infusion could lessen the post-operative incidence of insulin resistance, which may add to PONV [26, 27]. It also diminishes the outcome of night fasting, maintains hepatic glycogen, diminishes the stress, and enhances the insulin sensitivity of tissues [13, 28].
In addition, as known as post-operative pain is a known risk factor for PONV [29], hyperglycemia may raise plasma cholecystokinin, which can enhance pain and anxiety through its functions inside the brain, consequently decreasing pain and thus decreasing PONV [30, 31].
On the other hand, Patel et al. [32], in his study found an incidence of 52.9% in dextrose group and 46.7% in the control group. This may be because of administration less dextrose for a prolonged period (5% dextrose 250 ml for 2 hours).
In addition, another study done by McCaul et al., reported that administration of dextrose containing IV fluids was not effective in preventing PONV when compared to dextrose free IV fluid supplemented after elective gynecological laparoscopy [33]. Patients in their study received a relatively large volume of Glucose in a shorter duration of anesthesia (less than 25 minutes) than in our studies. The administration of large amounts of dextrose during a short surgery with an otherwise low risk of PONV may just increase gastric emptying and gastric fullness consequent to hyperglycemia.
Hyperglycemia now is believed to be associated with the increased risk of many complications such as dehydration, electrolyte imbalance, fluid shifts, ketoacidosis, and hyperosmolar states. It also increases the risk of morbidities thus increase the length of hospital stay during the post-operatively after surgeries and also mortality [30, 34].
The other main finding was that the postoperative blood glucose levels in (group D) patients were slightly higher compared to the (group RL) though the blood glucose level in all participants was within the normal range.
This result is in parallel with the study done by Firouzian et al., at which the patients undergoing LC was given Dextrose 5% 30 minutes pre-operatively and had a significant elevation in blood sugar level than the control group but within the normal range [15].
These results matched also with Patel et al., who gave the patients intravenous dextrose during the emergence of anesthesia and had elevated blood glucose level than the control group in the study [32].
On the contrary to the current study, Atashkhoei et al., (2018) used intraoperative dextrose 5% in patients undergoing Diagnostic Gynecologic Laparoscopy and the level of blood glucose were not different between the two groups [21].
Libiszewski et al., also concluded that changes in the level of blood sugar were not significantly different after preparation of patients with oral glucose solutions [32].
This study has several limitations. First, we assessed the effect of administration of IV dextrose for the prevention of PONV in non-smoking, healthy female patients who had undergone LC (which represent a high-risk group). Our results may not be generalizable to other populations including patients who undergo surgeries of different types or duration (as well as use different types of anesthesia). Comparing the effects of different concentrations of dextrose might be of interest in the future.
Undoubtedly, using this method for the prevention of PONV in diabetic patients is, while initially promising, debatable and needs more research. In addition, we did not assess postoperative pain as a risk factor for PONV in this study; as all patients received the same postoperative analgesic regimen. In addition, the tonicity of the tested fluids was not similar, thus their distribution inside the body is not the same.
Finally, the results might be affected by unknown variables. However, we attempted to match known confounding factors such as female sex, younger age, history of PONV or motion sickness, non-smoking, general anesthesia, utilizing N2O, perioperative fasting, ASA physical status and span of anesthesia.
LIST OF ABBREVIATIONS
| PACU | = Post Anesthesia Care Unit |
| ECG | = Electrocardiogram |
| PEEP | = Positive End Expiratory Pressure |
| IAP | = Intra-Abdominal Pressure |
| HR | = Heart Rate |
| VDS | = Verbal Descriptive Scale |
| LR | = Lactated Ringer |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The work was approved by the Research Ethics Committee of ASUHs (FMASU R 43/ 2018).
The study was prospectively registered with Pan African Clinical Trial Registry (PACTR) with Registration Number PACTR201808847015879 and conducted in the period from August 2018 to October 2018.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from ASA physical status I and II nonsmoker female patients, 20-40 years of age.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


