All published articles of this journal are available on ScienceDirect.
Using the BBraun BSmartTM Pressure Manometer to Prevent Unsafe Injection Pressures During Simulated Peripheral Nerve Blockade: A Pilot Study
Abstract
Background:
Peripheral nerve injury during regional anaesthesia may result from accidental intraneural placement of the needle, or forceful needle nerve contact. Intraneural injections are associated with increased resistance to injection, typically >15 psi. The BBraun BSmart™ is an inline mechanical manometer, offering a visual display of injection pressures.
Objective:
The primary objective of this study was to determine if using the BBraun BSmartTM manometer successfully prevents 90% of anaesthetists and anaesthetic assistants from injecting at pressures > 15 psi during simulated nerve block.
Methods:
This was a prospective observational study involving anaesthetists and anaesthetic assistants. Two 20 ml injections were performed by each participant, once when the BBraun BSmartTM manometer was obscured from view, and once with the manometer visible. A PendoTech PressureMATTMS recorded injection pressures.
Results:
39 participants completed the study, with a total of 78 injections recorded. During the study, 32 peak pressures during the 78 procedures were recorded above the recommended upper limit of 15 psi, 41% of the total injections. The peak pressure rose above 15 psi in 24/39 (62%) injections when the BBraun Bsmart™ manometer was obscured, but only in 8/39 (21%) injections when the manometer was visible.
Conclusion:
The BBraun Bsmart™ manometer did not successfully prevent 90% of anaesthetists or anaesthetic assistants from injecting at unsafe pressures. However, using the BBraun BSmart™ did reduce the number of unsafe injection pressures generated by participants. When utilised in conjunction with PNS and ultrasound guidance, this may offer additional safety during peripheral nerve blockade.
1. INTRODUCTION
Regional anaesthesia, specifically peripheral nerve blockade (PNB), is an increasingly popular method of providing both anaesthesia and analgesia during the peri-operative period. Custom-designed regional anaesthesia needles are used to perform PNB, an example is shown in Fig. (1). They are manufactured with integrated injecting tubing connected to a hollow needle, to facilitate remote injecting whilst being able to keep the needle still. These echogenic needles are easily visible on ultrasound. When performing PNB, the intention is for the anaesthetist to place the needle tip immediately adjacent to the target nerve, then deposit local anaesthetic alongside the nerve [1]. This is described as a perineural or extraneural injection. The needle should not make contact directly with the nerve, to reduce the risk of peripheral nerve injury (PNI).
The incidence of permanent PNI resulting from regional anaesthesia is fortunately rare, quoted as 2-4 per 10 000 nerve blocks [2]. Whilst transient neurological symptoms are common after PNB, the vast majority resolve with time [2]. Disappointingly, this incidence has not declined in the last decade since the introduction of ultrasound guidance into routine clinical practice [2].
Nerve fascicles are bundles of nerve axons that run together, bound by the perineurium within a peripheral nerve. The perineurium offers the vulnerable fascicles physical and chemical protection. It prevents potentially toxic substances from diffusing into the nerve, maintaining the internal environment of the fascicle [2].
Accidental placement of the needle tip within the nerve during PNB is termed an intraneural injection. An intraneural injection may be sub-classified as intrafascicular or extrafascicular, depending on the exact location of the needle tip within the nerve structure. An intraneural injection may also be innocuous, without subsequent PNI: the delicate fascicles occupy only one-quarter of the cross-sectional area of a peripheral nerve, with the rest comprising of connective tissue [3]. It is possible that a needle tip inserted beneath the perineurium does not contact a fascicle, therefore avoiding mechanical disruption. The image resolution of commercially available ultrasound equipment is not detailed enough to distinguish between intra- and extra-fascicular needle tip placement.
PNB has not been identified as an independent risk factor for peri-operative nerve injury [4]. However, the American Society of Regional Anesthesia (ASRA) committee maintains that deliberate needle-nerve contact (NNC), intraneural needle insertion, and intraneural injection should all be avoided due to the potential risk of PNI [2].
To date, three methods of identifying the exact location of peripheral nerves for PNB have been utilised in clinical practise:
1.1. Paraesthesia
Forceful NNC or nerve penetration may elicit paraesthesia. Due to the risk of nerve damage, this technique of nerve identification has largely been abandoned [5]. The onset of pain or paraesthesia in a nerve distribution during PNB should alert the operator to withdraw the needle and cease injecting. Deliberate puncture of peripheral nerves does not invariably result in paraesthesia or dysaesthesia, which may contribute to inadvertent nerve injury [6].
1.2. Peripheral nerve Stimulation
Nerve localisation with a peripheral nerve stimulator (PNS) is based on the principle of Ohm’s law. A PNS relies on an inverse relationship between the current required to elicit muscle contractions, and the distance between the stimulating needle tip and the nerve supplying that muscle [1].
Traditionally, a threshold stimulating current of 0.2-0.5mA has been described as the optimal stimulating endpoint for PNB [7]. Currents less than 0.2 mA that elicit a motor or sensory response are presumed to be a result of intraneural needle placement. The requirement of currents greater than 0.5 mA to successfully generate a motor response implies that the needle tip is too far from the nerve to result in successful PNB.
Nerve stimulation as a technique of nerve localisation may be inaccurate as intraneural needle placement does not invariably result in nerve stimulation, regardless of the current strength [1, 6].
In 2006, a study established the sensitivity of paraesthesia as a method of detecting NNC as 38% [8]. The sensitivity of nerve stimulation (at a current of ≤ 0.5 mA) in identifying NNC is 74.5% [8]. This highlights the potential safety implications of clinicians limiting themselves to utilising a single method of needle location during PNB. A further study has shown that in 17% of patients, the intraneural location of the needle-tip does not generate a muscle response when using the PNS, even following an increase in current strength to 1.5mA [9].
1.3. Ultrasound Guidance
During PNB, ultrasound guidance allows real-time visualisation of the targeted peripheral nerve, needle tip, needle shaft and the injectate. Ultrasound-guided regional anaesthesia (USGRA) has been proved to be superior to PNS-guided PNB in the following ways: increased speed of onset of anaesthesia, the fewer needle passes, increased patient comfort, and lower volumes of injectate required [1]. However, it has not yet been shown to be superior in relation to patient safety [1]. The causes of unintentional intraneural injection during USGRA are either an inability to identify the needle tip in real-time, or incorrect interpretation of the ultrasound image obtained [7, 10]. Current best practise recommends that a technique of hydro-dissection is used in conjunction with ultrasound guidance [7]. In this technique, small volumes of local anaesthetic are injected multiple times during PNB. The injectate creates a path for the needle tip to be advanced safely into. This results in contact of the injectate, but not the needle tip, with the desired nerve [7].
At present, no single method of detecting needle tip placement can be considered infallible, or superior to another. This is reflected in the comment from ASRA: “There are no human data to support the superiority of one nerve localisation technique over another with regard to reducing the likelihood of peripheral nerve injury.” [2].
Various methods of detecting and preventing intraneural injections have been described:
1.4. Subjective “Syringe Feel”
Typically, the main operator performing the PNB manipulates the needle while observing the ultrasound image, and an assistant performs the injection of local anaesthetic. If the needle tip is placed intraneurally, the assistant should sense increased resistance when depressing the syringe plunger, as the rigid perineurium resists expansion. If the assistant does not cease injecting, this higher resistance is followed by a sudden loss of tension, as the integrity of the fascicles and connective tissue is disrupted [11].
Claudio et al. confirmed there is significant variability amongst experienced clinicians in perceiving “normal” injection pressures, and that this subjective method is both inaccurate and inconsistent [12]. High injection pressures may inadvertently be generated when using small volume syringes. Clinicians should routinely use large volume syringes (20 ml) when performing PNB as these magnify the tactile feedback if a greater force is required to inject [13].
1.5. Commercially Available Inline Manometers
The BBraun BSmartTM injection pressure manometer (BBraun Melsungen AG, Melsungen, Germany) is a commercially available mechanical pressure monitor. The BBraun BSmart™ is intended to reduce inter-individual variability and facilitate rapid termination of injection when high pressures are detected during PNB. The device is pictured in Fig. (2).

The single-use device is placed in-line between the syringe and injection tubing, prior to the entire system being primed with injectate. Fluid from the syringe passes through the pressure monitor, into the extension tubing, and into the needle. The manometer has a piston, which is forced upwards during injection, proportionately to the pressure generated during the injection. The piston is colour-coded. Injecting into compliant tissues will cause the piston to rise up to display the white bar on the piston (< 15 psi). Increased resistance results in the piston rising higher to display the yellow bar (15-20 psi). Pressures greater than 20 psi expose the red bar on the piston. This may be due to NNC intraneural injection, or the needle lying against bone, tendons or fascial planes. If this is detected during clinical use, injecting should cease immediately, and the needle repositioned. An increase in pressure detected during PNB injection is not always a result of incorrect needle tip placement. Injection pressure may be elevated as a result of the speed of injection, the dimensions of the needle and the length of integrated injection tubing. [14, 15].
Gadsden et al. performed a prospective observational study in patients undergoing elective arthroscopic shoulder surgery with interscalene brachial plexus PNB [10]. Deliberate NNC consistently generated injection pressures above 15 psi. When the needle tip was greater than 1mm from the nerve root, opening injection pressures were persistently less than 15 psi, with a mean peak pressure of 8.2 psi. Therefore, the authors recommend that opening pressures greater than 15 psi during PNB should result in immediate discontinuation of injection. Similar studies performed on cadavers confirm these pressure limits. Krol et al. confirmed injection pressures of 29.4 ± 9.3 psi during intraneural injections, compared to 7.2 ± 2.5 psi during extraneural injections [16]. Vermeylen et al. found deliberate intraneural injections resulted in opening injection pressures (OIP) of 21.5-25.8 psi. Perineural injections resulted in a lower OIP of 3.8-6.1 psi [17]. To date, there are no cases published that report clinically significant neuropathy in the presence of low injection pressures. Thus, injection pressure monitoring may prove most useful as a negative predictor of PNI [18].
Increasing the speed of injection generates higher flow rates. This leads to an increase in pressure values recorded by inline pressure monitors. However, it is a result of the inherent resistant in the needle and connecting tubing, rather than resistance at the needle tip [15]. Patil et al. recommend that injection flow rates should not exceed 15 ml/min (0.25 ml/s) [11].
2. OBJECTIVES
Peri-operative PNI is the third most common cause of litigation related to anaesthetic practise [19]. Permanent nerve damage following PNI may result in a devastating loss of function and chronic pain in the affected limb, with a significant reduction in the patient’s quality of life [20].
Studies have validated the BBraun BSmart™ as an accurate digital pressure manometer at identifying high injection pressures [21]. However, the literature has not yet confirmed if the BBraun BSmart™ does indeed successfully prevent anaesthetists or anaesthetic assistants from injecting at unsafe pressures.
The primary objective of this study was to determine if the use of the BBraun BSmartTM manometer successfully prevents 90% of anaesthetists and anaesthetic assistants injecting at pressures of greater than 15 psi during simulated PNB. Secondary objectives included the measurement of peak injection pressures (psi) generated during simulated PNB, and average flow rates (ml/min) during simulated PNB.
3. MATERIALS AND METHODS
This was a prospective observational study, carried out at a central London teaching hospital.
Inclusion criteria consisted of the following: permanent employees, anaesthetists and anaesthetic assistants, and familiarity with performing injections for PNB. Anaesthetic assistants may be either Operating Department Practitioners (ODPs) or Anaesthetic Nurses (ANs).
Exclusion criteria consisted of the following: refusal to participate in the study, loss of capacity to consent to the study, unfamiliarity with performing injections for PNB, physical inability to depress the syringe plunger, inability to visualize the BBraun BSmartTM piston.
The first part of the study involved sourcing and purchasing the equipment we required in order to display, and record, the injection pressures generated during simulated PNB.
The company representative from BBraun was contacted. BBraun kindly donated 10 BBraun BSmartTM pressure monitors to be used in the study. These have a commercial value of approximately £5 each.
A PendoTECH pressure sensor and PendoTech PressureMATTMS were purchased from the United States (PendoTECH, Princeton, New Jersey). These two electronic items are designed to be used in conjunction with each other. The pressure sensor is connected simultaneously to the PendoTECH PressureMATTMS, and in-line with the syringe and PNB needle extension tubing. The MATTMS functions as a pressure monitor, high-pressure alarm, and a transmitter [22]. According to the manufacturer’s website, the portable, lightweight monitor does not need calibration or maintenance [22]. The PressureMATTMS has a digital display where the process pressure can be read in either kPa or psi. There is a button to zero tare to atmospheric pressure prior to each measurement. Fig. (3) shows a PendoTECH PressureMATTMS displaying a pressure measurement in psi.
The next step of the study involved assembling the equipment and simulating a PNB with the study participants. Two sets of study equipment were assembled.
The first set was used to demonstrate the correct use of the BBraun BSmartTM manometer in clinical practise: A 20ml syringe was connected to the BBraun BSmartTM manometer, which in turn was connected to the extension tubing of a 100 mm Pajunk Stimuplex Ultra® regional anaesthesia needle. The syringe contained water. This set of equipment was not used to gather data during the experiment, only to facilitate candidate’s practise using the BBraun BSmartTM.
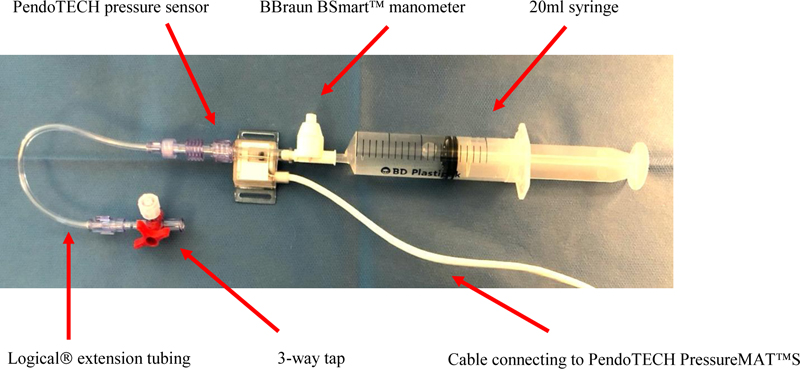
The second set of equipment was used to gather the study data. Regional anaesthesia needles were not required for this stage of the study. A 20 ml syringe was connected to the BBraun BSmartTM manometer, which in turn was connected to PendoTECH pressure sensor. The PendoTECH pressure sensor cable was in turn connected to the proximal end of extension tubing of a LogiCal® invasive pressure monitoring kit. This extension tubing was used in place of the extension tubing of a PNB needle: The distal end of the LogiCal® extension tubing has a three-way tap which the study team could secure in a partially closed position. By creating a constant restriction to flow during injection, we simulated an increased resistance during PNB injection. Fig. (4) shows the assembly of study equipment.
The PendoTECH pressure sensor was connected to the PressureMATTMS and coupled to a computer. This allowed digital recordings of the injection pressures (psi) and to record the duration of injection during simulated PNB.
In summary, the equipment assembly was: Syringe - BBraun BSmartTM - PendoTECH pressure sensor - extension tubing - three-way tap.

For each candidate, the principal investigator (PI) performed a demonstration of simulated PNB. A 20 ml syringe filled with water was attached to the BBraun BSmartTM inline pressure monitor, which in turn was connected to the proximal end of extension tubing of a peripheral nerve block needle. The PI flushed the system with water, then performed a simulated injection, demonstrating how the BBraun BSmartTM monitor’s piston works. This was done by the PI manually occluding the extension tubing to simulate increased resistance. The BBraun BSmartTM colour coding and pressure readings were explained to each participant. The clinical relevance of these pressures was discussed, notably that injecting should cease at pressures above 15 psi. Following this demonstration, participants were allowed to perform a single practice injection whilst being able to see the BBraun BSmart TM inline pressure monitor.
Following this, the study equipment was assembled as described above, with the PressureMATTMS digital display obscured from the candidates. The participants were asked to imagine a hypothetical clinical scenario where they were assisting a colleague in performing regional anaesthesia and were responsible for administering the local anaesthetic injection. Participants were asked to inject 20 ml saline into a beaker on two sequential occasions. They were to do this at the rate and pressure that they would normally use in their clinical practice when performing PNB. Candidates were requested to aspirate every 5 ml, as is common in clinical practise to exclude intravascular injection. Thus, injecting 20 ml took place in 4 phases of 5 ml injections.
The first 20 ml injection was performed with the participant unable to see the piston of BBraun BSmartTM, it was obscured by a piece of cardboard. The PressureMATTMS recorded the pressures in psi generated during each phase of the injection, and the total duration of time in seconds over which the injection was performed. The PendoTECH PressureMATTMS is programmed to sample process pressures every second.
The second 20 ml injection was then performed with the participant able to see the BBraun BSmartTM piston. The PressureMATTMS again recorded the pressures generated during each phase of the injection, and the time over which the injection was performed.
3.1. Ethics Approval
The study was approved by the Faculty of Medicine and Health Sciences Ethics Committee at the University of Dundee. Ethical approval was obtained successfully on 19 August 2019 (Ref 201819-143).
All participants provided written consent to participate in the study. All measurements were recorded by the PI onto a spreadsheet (Microsoft® Excel® for Mac 2011 version 14.0.0), having been anonymised prior to recording. Data was managed in accordance with the General Data Protection Regulation (GDPR) and Data Protection Act 2018.
3.2. Statistical Analysis
Statistical tests were chosen prior to the commencement of the study, following advice from a statistician (JW). Calculations were performed using professional statistical software (Stata® 16, StataCorp LLC). The study was designed to assess the primary outcome: Does the use of the BBraun BSmart™ prevent 90% of anaesthetists and anaesthetic assistants from injecting at unsafe pressures? This outcome is categorical in nature. Categorical (nominal and ordinal) variables have been presented as n (%), continuous variables as mean (SD). Demographic data were recorded on an Excel spreadsheet (Microsoft® Excel for Mac Version 16.31).
The sample size was determined to be a minimum of 34 participants. This was based on preliminary equipment testing that suggested fewer than 5% of candidates using the BBraun BSmart™ monitor will inject at unsafe pressures.
Using exact binomial confidence intervals in Stata® 16, a group of 34 participants would include the 90% goal, with a Type 1 error of 5% (p < 0.05). Additionally, we performed sensitivity analyses under pessimistic hypotheses. This confirmed the study size would still have a power greater than 80% to detect a benefit of the BBraun BSmart™ over unmonitored injecting.
The primary outcome was tested using the Binomial Test, an exact test for binomial random variables. Unequal test tail areas were specified due to the safety-oriented nature of the study and the asymmetric distribution of the binomial distribution at extreme values. The Type I error was constrained to 5%.
The secondary outcomes of the study explored the average flow rates during injection (ml/min) and the peak pressures generated during injections (psi), generated by the participants when able to see the BBraun BSmart™, and when unable to see the inline pressure manometer.
A paired t-test was performed to compare the peak pressures (psi) and the mean flow rates (ml/min), as we were comparing a continuous variable between the two groups.
During recruitment, this sample size was increased to 40 to allow sufficient data recordings in the event of dropout or technical difficulties. During the study, the pressure and flow recordings of a single participant were lost due to a programming failure. This resulted in 78 pressure waveforms being obtained for the study team to analyse.
4. RESULTS
Forty participants were recruited in total. For one volunteer, the PendoTECH software did not record any pressure-time data, so was excluded from the subsequent analysis.
Two pressure-time curves for each of the 39 candidates were available for analysis. The pressures generated during simulated injection were recorded at a sampling rate of 1 per second (1Hz).
The first pressure-time curve was recorded with the candidate injecting 20 ml saline, whilst unable to see the BBraun BSmart™ manometer. During the second injection of 20ml, the participant was able to visualise the manometer.
The study group consisted of 23 (59%) women and 16 (41%) men. Regarding occupation, there were 26 (67%) anaesthetists and 13 (33%) Operating Department Practitioner (ODPs) or Anaesthetic Nurses (ANs).
Table 1 below displays the number of years of clinical experience for each of the candidates. In the case of ODPs/ANs, this refers to the time since qualifying in this field. For anaesthetists, this is taken as the time since entering the specialty of anaesthesia.
Table 2 below displays the estimated number of PNBs assisted or performed per year for each candidate.
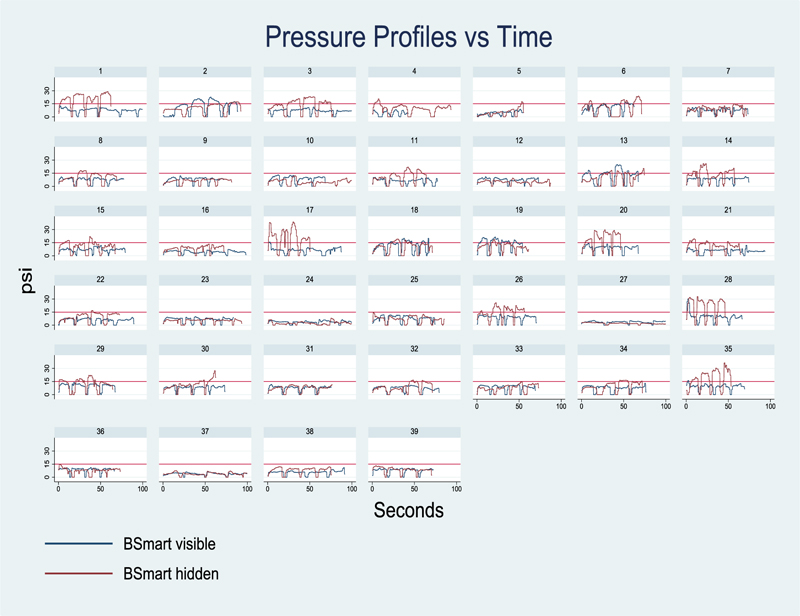
Pressure-time curves were constructed for each candidate. An overview of the curves for all participants is displayed below in Fig. (5). For each candidate, the red line represents the first phase of the study with the BBraun BSmart™ manometer obscured from view. The blue line represents the second 20ml injection, with the BBraun BSmart™ manometer visible to the candidates. In clinical practice, the 20 ml syringe was injected in 5 ml aliquots, resulting in four pressure peaks and four pressure troughs.
| - | 0-4.9 y Qualified | 5-9.9 y Qualified |
10-14.9 y Qualified | 15 – 19.9 y Qualified | >20 y Qualified |
|---|---|---|---|---|---|
| Number (%) of candidates | 5 (13) | 11 (28) | 9 (23) | 6 (15) | 8 (21) |
| - | < 10 PNBs/Year | 10-19 PNBs/Year | 20-39 PNBs/Year | ≥ 40 PNBs/Year |
|---|---|---|---|---|
| Number (%) of candidates | 2 (5) | 12 (31) | 13 (33) | 12 (31) |
During the study, a total of 32 peak pressures during the 78 procedures (BBraun Bsmart™ manometer visible and non-visible) were recorded above the recommended upper limit of 15 psi. This is 41% of the total injections performed by the candidates. Of these 32 episodes, 19 (59%) injections reached pressures above 20 psi. A total of nine (28%) injections generated pressures greater than 25 psi.
The peak pressure rose above 15 psi in 24/39 (62%) injections when the candidates were unable to see the BBraun Bsmart™ manometer but only in 8/39 (21%) injections when the manometer was visible (p = 0.0004 Fisher’s exact test). With a null hypothesis that the BBraun BSmart™ device would not prevent 90% of subjects injecting at pressure over 15 psi, an exact binomial test was performed, which gave a one-sided p-value of 0.037. This makes the observed data consistent with the null hypothesis. In this study, we set out to evaluate if the BBraun Bsmart™ does indeed successfully prevent 90% of anaesthetists or anaesthetic assistants from injecting at unsafe pressures. These results, therefore, demonstrate a negative finding for the primary outcome of our study.
A total of four pressures greater than 30psi were recorded, generated by three study participants. Three of four (75%) of these took place when the BBraun BSmart™ was hidden from the candidates. One candidate reached a peak pressure of 38.8 psi during the first injection, and a peak pressure of 32.8 psi while completing the second.
Using the BBraun BSmart™ did not result in a universal reduction of peak injection pressures from the first to the second injection, although this was a more common finding. For ten candidates, the peak injection pressure was higher when the BBraun BSmart™ was visible to them during the second phase of the study.
4.1. Reduction in Peak Pressure
There was a total of 29 cases where the peak pressure was lower during the second injection, with the BBraun BSmart™ manometer visible, than during the initial injection. Of these 29, in 25 cases, the second injection was performed with a peak pressure below 15 psi. For four candidates, although there was a reduction in peak pressure, the pressure generated remained above the safety threshold of 15 psi.
Of the 25 subjects in which the second peak pressure was below 15 psi, 16 candidates had initially injected at pressures above 15 psi. Thus, in 16 candidates, the BBraun BSmart™ assisted in reducing peak pressures from an unsafe range into a range considered safe in clinical practise.
The mean (SD) psi for the group of candidates during the initial injection was 18.7 (8.0) psi. During the second injection, when the BBraun BSmart™ was visible, it was 12.9 (5.1) psi which is a reduction of 5.8 psi (p < 0.0001, 95% CI 3.5 – 8.1), assuming normally distributed variables.
4.2. Increase in Peak Pressure
In the remaining ten participants, with the BBraun BSmart™ being visible, there was an increase in the peak pressure recorded during the second injection. In six cases, despite a rise in injection pressure, the peak pressure recorded remained below 15 psi during both phases of the study. However, in four cases, with the BBraun BSmart™ visible, injections pressures exceeded the 15 psi limit.
4.3. Flow Rate
In addition to an assessment of the peak pressures generated during the study, a further secondary outcome was the average flow rate during injections. Flow rates below 15 ml/min or 0.25 ml/s are recommended [11, 16]. During our study, a total of 61/78 (78%) injections were delivered at speed greater than 15 ml/min (0.25 ml/s).
Regarding the first 20 ml injections, the total time taken to inject 20 ml of saline ranged from 42-135 seconds. Thirty of 39 (77%) candidates exceeded the recommended injection speed of 15 ml/min (0.25 ml/s). The greatest speed of injection reached by a candidate was 0.48 ml/s, the slowest flow rate was 0.15 ml/s.
During the second set of injections, the total time taken to inject 20 ml of saline ranged from 34-105 seconds. Thirty-one of 39 (79%) candidates exceeded the recommended injection speed of 15 ml/min (0.25 ml/s). The greatest speed of injection reached by a candidate was 0.6 ml/s, the slowest flow rate was 0.19 ml/s.
For the initial phase, when the BBraun BSmart™ was obscured from the candidates, the mean (SD) flow rate was 0.313 ml/s (0.011), with a 95% CI 0.290-0.336. When able to see the BBraun BSmart™ the mean (SD) flow rate was 0.293 ml/s (0.07) with a 95% CI 0.271-0.316. The difference in flow rate between observed and non-observed groups was non-significant (p 0.17).
5. DISCUSSION
The use of the BBraun BSmart™ inline pressure manometer during simulated PNB injection did not prevent 90% of anaesthetists and anaesthetic assistants from injecting at pressures above 15 psi.
This result was unexpected. We had anticipated that the BBraun BSmart™ would be simple to use and result in 100% of simulated PNB injections being performed within a pressure range considered to be safe (<15 psi). The colour markings on the piston of the BBraun BSmart™ provide immediate visual feedback during simulated PNB and eliminate the subjective nature of “syringe feel.” In our study, despite the use of the BBraun BSmart™, 21% of participants injected at pressures > 15 psi during the second phase of the experiment. However, while this does not achieve our 90% aim, it is still a marked improvement on the first set of injections performed by the participants. During the first phase, 62% injections were above 15 psi. These results are similar to those obtained during a benchtop study by Claudio et al. [12]. When this group evaluated the accuracy of “syringe feel” during simulated PNB, they found 70% of subjects injected at pressures > 20 psi.
It is worth contemplating the reasons why using the BBraun BSmart™ did not successfully modify the behaviour of all anaesthetists and anaesthetic assistants. The possibility of the device being too complex to understand does exist. However, it is a simple piece of equipment. In addition, the PI explained how the manometer worked, demonstrated it to the candidates, and then gave the candidates the opportunity to use it prior to performing the study. A second possibility is that the manometer is slow to respond to increases in pressure, so there is delay before the candidates recognise the increase in pressure and respond. This is unlikely as it is a free-moving piston and is in-line with the syringe. Thirdly, it may be a result of the artificial nature of the study and the brief given to the participants. As a result of informing them we were calibrating the equipment, rather than explaining the true intention of the study, participants may have been less cautious when injecting than in a clinical setting.
Patil et al. noted that pressure monitors placed upstream from the needle, such as the BBraun BSmart™, may produce false-positive readings during higher flow rates of injection [23]. These false-positive readings are caused by the flow characteristics of the injectate changing from laminar to turbulent flow during rapid injection, or the inherent resistance to flow from the needle shaft [23]. Their study recommends restricting flow rates to ≤15 ml/min to reduce the likelihood of false-positive pressure readings due to proximal factors. This is especially applicable when using custom-designed PNB needles such as the Stimuplex Ultra® in our study (BBraun Melsungen AG, Melsungen, Germany). These needles have integrated plastic extension tubing, which further increases the resistance of the system.
In our study, there was little difference in the average flow rates between the two phases of injecting. During both phases, over three-quarters of candidates exceeded the recommended flow rate of 15 ml/min. This is greater than the proportion in the study by Patil et al., in which fifty percent of their cohort were noted to inject at rates above the recommended 15ml/min or 0.25 ml/s [11]. This is another unexpected finding. We had assumed that the use of the BBraun BSmart™ would result in participants injecting with greater caution, at a slower rate.
While there are concerns regarding too high flows during PNB, it is equally possible that a missed intraneural injection may result from injecting at a much slower speed, where there is a very slow rise in pressure.
5.1. Limitations of our Study
This was a benchtop study and did not involve human volunteers or patients. A clinical setting may have impacted the results we obtained. Our participants may have been less cautious when performing the simulated PNB than they would be in a clinical setting, as there was no possibility of causing patient harm. Alternatively, knowing they were being observed by a colleague, there may have been a “halo effect” during our study, with participants acting over-cautiously. Through the use of minor deception to the candidates, unnatural behaviour was likely to be minimal.
A second limitation is unfamiliarity with the BBraun BSmart™. None of our participants had previously routinely used the BBraun BSmart™ in clinical practise. However, it is a simple piece of equipment and should not require in-depth training prior to use. It is marketed for its simplicity.
Thirdly, the BBraun BSmart™ is designed for single use as an infection control precaution. Due to the limited samples, each manometer was used a number of times. It is not known if the accuracy of the BBraun BSmart™ deteriorates with repeated use. It is possible that the piston becomes less free to move with multiple injections, although this was not obvious to the study team. No piston ceased to function during the study.
We created a constant flow restriction by means of a partially occluded three-way tap to simulate increased resistance to injection. This is similar to an intraneural injection in that there would be initial high resistance to injection. However, in a true intraneural injection, following disruption of the nerve structure, the resistance to injection would immediately decrease. We were not able to vary the flow restriction during the study, as we needed to ensure the initial resistance to injection was consistent between candidates.
Finally, due to limitations of the PendoTech PressureMATTMS software, we were only able to sample pressures every second. As a result, we may have missed peak pressures that occurred between this sampling frequency.
5.2. Strengths of our Study
This was an important study to undertake as it explores the possible overreliance of clinicians on simple safety tools. It adds weight to the argument of incorporating more than one method to locate nerve during PNB.
All participants were familiar with the process of PNB. Only 5% of candidates performed less than 10 PNB per year.
Through utilising a small element of deception, the candidates were not aware that their pressure and flow measurements were being recorded. They had been informed that the expensive PendoTech PressureMATTMS was being calibrated with the inexpensive BBraun BSmart™. This relieved any stress from being observed by a fellow colleague, allowing them to inject in the manner that they would in a clinical setting. In addition, participants were requested to inject as they would in the clinical setting, aspirating every 5 ml. In this way, the benchtop study was as close to clinical practise as possible.
5.2.1. What our Study Contributes to Current Understanding
This study adds to our current knowledge base. It demonstrates that the BBraun BSmart™ does result in a lower proportion of anaesthetists and anaesthetic assistants injecting at unsafe pressures, and at slightly slower injection rates. It highlights the fact that the usefulness and success of the BBraun BSmart™ is limited by behaviour modification of the practitioner performing the PNB.
As the practise of medicine becomes increasingly litigious, documenting the pressures generated during PNB may be of benefit. In the unlikely but devasting event of a PNI, the use of pressure monitoring may demonstrate adherence to best clinical practise [20]. Documentation of peak pressures obtained during injections may also encourage clinicians to limit their pressures during PNB injections.
However, to date, widespread implementation of the BBraun BSmart™ has not been recommended in the literature, until there is more evidence to support its use [14]. Whilst the BBraun BSmart™ does provide an objective and reliable method of measuring injection pressures during simulated PNB, it is important to acknowledge that it does not actually limit the injection pressure. Clinicians should not be overly reassured by this piece of equipment. In addition, it cannot differentiate between proximal and distal causes of increased resistance to injection.
Our study reaffirms there is no infallible method of limiting pressure injections during PNB. The BBraun BSmart™ has been shown to be vulnerable to human error and misuse. The manufacturer’s website recommends the technique of “Triple Monitoring” which incorporates the simultaneous use of ultrasound, PNS and injection pressure monitoring during PNB to reduce the risk of intraneural injection [24]. We support this recommendation.
CONCLUSION
Using the BBraun BSmartTM pressure, the manometer did not fully prevent unsafe injection pressures being generated during simulated PNB. However, 79% of anaesthetists and anaesthetic assistants injected only at safe pressures when able to view the BBraun BSmart™ pressure manometer. When utilised in conjunction with PNS and ultrasound guidance, this may offer additional safety to the patient.
Measurement of injection pressures may be particularly useful when the image obtained on ultrasound is suboptimal, or as an adjunct when trainees are performing PNB [10]. Additionally, it may also be of some reassurance to anaesthetists to have injection pressures objectively monitored when not self-administering the local anaesthetic [11].
We recommend incorporating the BBraun BSmart™ manometer as part of routine clinical care when performing PNB to reduce the rare yet devastating occurrence of PNI. Perhaps in using a combination of imperfect methods, we will be able to offer the greatest safety during PNB: “Safety is a mix of proper training, reliable monitors, good judgement, and plain old common sense [25].”
ETHICAL STATEMENT
The study was approved by the Faculty of Medicine and Health Sciences at the University of Dundee. Ethical approval was obtained successfully FMH Research Ethics Committee, University of East Anglia, on 19 August 2019 (Ref 201819-143).
The PI is registered with the Information Commissioner’s Office and complied with the GDPR Code of Conduct 2018 when processing the study and demographic data.
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Participants were not informed of the true aim of the study. An element of minor deception was employed, approved by the Ethics Committee. It was felt necessary as this was a study exploring behaviour modification when using a piece of equipment. If the candidates had been aware of the true aim, it may have affected the results obtained. Participants were informed that we were calibrating the inexpensive BBraun BSmartTM with the expensive PendoTECH PressureMATTMS to assess for accuracy in the single-use device.
STANDARD OF REPORTING
STROBE guideline has been followed in this study.
FUNDING
The purchase of the PendoTECH PressureMATTMS was funded by the hospital charity (CW+ of Chelsea and Westminster Hospital).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank BBraun for donating the ten BBraun BSmartTM manometers, and the hospital charity CW+ for the purchase of the PendoTECH PressureMATTMS.
Ten BBraun BSmartTM pressure monitors were donated to the hospital by the BBraun industry representative, each with a commercial value of approximately £5.
LIST OF ABBREVIATIONS
| AN | = Anaesthetic Nurse |
| ASRA | = American Society of Regional Anaesthesia |
| NNC | = Needle-Nerve Contact |
| ODP | = Operating Department Practitioner |
| OIP | = Opening Injection Pressure |
| PI | = Principal Investigator |
| PNB | = Peripheral Nerve Blockade |
| PNI | = Peripheral Nerve Injury |
| PNS | = Peripheral Nerve Stimulator |
| PSI | = Pounds per Square Inch |
| USGRA | = Ultrasound-Guided Regional Anaesthesia |


