Opioid-free Anesthesia for Laparoscopic Gastroplasty. A Prospective and Randomized Trial
Abstract
Background:
Opioid-free anesthesia (OFA) has gained popularity in recent years due to concerns about the abusive use of this drug but also due to the potential benefits of OFA for pain control and decreased side effects.
Objectives:
This trial aimed to study whether opioid-free anesthesia (OFA) benefits patients submitted to laparoscopic gastroplasty compared to anesthesia with fentanyl. The primary objective was to measure pain score and morphine use for rescue analgesia. The secondary objective was to evaluate the incidence of postoperative nausea and vomiting (PONV) and oxygen desaturation.
Methods:
Patients undergoing gastroplasty were randomized to receive general anesthesia with fentanyl (n = 30) or OFA (n = 30) according to a predefined protocol. They were assessed for pain using a verbal numerical scale (VNS), morphine consumption and PONV in the post-anesthesia care unit and on the first day after surgery. Besides, oxygen desaturation during the immediate postoperative period was also recorded. The study was blinded to the surgeon and postoperative evaluators.
Results:
The groups were comparable for all demographic data analyzed. A significance level of 5% was used, and no differences were found in the variables studied.
Conclusion:
The specific OFA protocol presented in this trial was safe and effective. However, this study did not find any benefit in using it compared with fentanyl anesthesia in videolaparoscopic gastroplasties.
1. INTRODUCTION
Opioids are considered the cornerstone drug of all general anesthesia, and their use for intraoperative and postoperative analgesia has increased in recent decades. Their chronic use has become increasingly common; concerns about their side effects and high addictive power have increased [1].
These drugs are known to cause adverse patient health, such as respiratory depression, urinary retention, nausea and vomiting, pruritus, and decreased intestinal motility, which is particularly important in the postoperative period and may cause delayed hospital discharge. Studies showed an association between decreased use of opioids in the perioperative period, better surgical outcomes, faster return of bowel function and oral intake, and earlier hospital discharge [2, 3].
Two other important adverse effects of this class of drugs (tolerance and hyperalgesia) have gained prominence in recent years, resulting in increased demand for analgesics after previous opioid use. Although they present different pathophysiological mechanisms, they are clinically difficult to distinguish [4]. Anesthesia with high doses of opioids may be related to increased pain and demand for analgesics in the postoperative period [5, 6].
The practice of anesthesia with lower doses of opioids using other types of drugs for multimodal analgesia has emerged due to these outcomes. This anesthesia denominated opioid-sparing became one of the focuses of the Enhanced Recovery After Surgery protocol (ERAS), created to optimize postoperative recovery [7]. Therefore, opioid-free anesthesia (OFA) was developed based on this concept.
This study aimed to analyze whether the use of the OFA protocol in patients undergoing laparoscopic gastroplasty is beneficial, assessing the pain score and use of rescue morphine as the primary objective and the incidence of postoperative nausea and vomiting (PONV) and oxygen desaturation as a secondary objective.
2. MATERIALS AND METHODS
This study is interventional clinical research. Before any evaluation, this study was submitted for review and approval by the Research Ethics Committee of the Health Sciences Sector of the Federal University of Paraná (CEP/SD), CAAE 39513520.3.0000.0102 (plataformabrasil.saude.gov.br; appro val date 12/07/2020). Informed consent was obtained from all participants. The study followed the recommendations of the Consolidated Standards of Reporting Trials (CONSORT) and was retrospectively registered on the ReBec platform (Brazilian Clinical Trials Registry) from the World Health Organization registry network (RBR-4m5pdbf, date 03/25/2022).
The selected participants were adult patients with body mass index (BMI) ≥35 kg.m-2 who indicated elective laparoscopic gastroplasty (Roux-en-Y gastric bypass) performed by the same surgeon. Exclusion criteria were refusal to participate in the study, chronic pain, chronic use of analgesics, any condition or pathology that could affect pain perception, heart block or significant arrhythmias, or patients anesthetized differently from the proposed protocol or by a professional who did not participate in the study. The prior allergy to any medication was evaluated on a case-by-case basis.
Based on previous studies with a medium effect size [8, 9], it was calculated a sample size of 60 for power analysis of 80%. After being selected by the inclusion and exclusion criteria, the participants were randomly divided into two groups through block randomization (6 blocks of 10 generated by computer) right before entering the operating room. The groups were named “Fentanyl” (anesthesia with opioids) and “OFA.” The result of the randomization was known only to the anesthesiologist who participated in the procedure. The study was blinded to the surgeon and the other physicians who evaluated the postoperative period.
All patients treated by this surgical team underwent a hypnosis session the day before surgery as an additional possibility of analgesia and relief from stress and anxiety [10]. No anxiolytic or analgesia was administered preoperatively. After a venous puncture, all patients received an infusion of 250 mL of saline solution (SS) containing cefazolin (2 g), parecoxib (40 mg), dipyrone (2 g), omeprazole (40 mg), dexamethasone (10 mg), and droperidol (1.25 mg) for 10 min. Along with this solution, the OFA group also received magnesium sulfate (40 mg.kg-1), ketamine (25 mg), lidocaine (1.5−2 mg.kg1), and dexmedetomidine (0.5 mcg.kg-1). The Fentanyl group was administered fentanyl 2.5 mcg.kg-1 in bolus (Table 1). Subsequently, general anesthesia was performed with propofol until loss of consciousness and rocuronium 1.2 mg.kg-1. All patients were induced in a rapid sequence and ventilated only in case of desaturation or difficulty of intubation. The drugs administered were based on the ideal body weight (IBW), except sugammadex, in which the corrected body weight (IBW +40%) was used [11]. Anesthesia was maintained in both groups with sevoflurane at 0.9−1 minimum alveolar concentration (MAC) and repeated doses of rocuronium 10 mg every 40 min. Finally, ondansetron 4 mg was infused. All patients received local anesthetic infiltration (levobupivacaine 0.5% with vasoconstrictor) in the incision before the procedure. The pneumoperitoneum pressure ranged from 12 to 13 mmHg. An analgesic solution was prepared for patients in the OFA group with 450 mL of SS, 75 mcg of clonidine, 25 mL (2.5 g) of magnesium sulfate, and 25 mL of lidocaine 2%. A dose of 1 mL.kg-1.h-1 of this solution was maintained from the beginning of the procedure until discharge from the post-anesthesia care unit (PACU). The Fentanyl group received an SS 500 mL infusion, so the study was blinded to the evaluators. The discharge from PACU occurred with an Aldrete−Kroulik index ≥9 and with a pain score by verbal numerical scale (VNS) ≤4. On the ward, all patients were administered dipyrone 1 g 6/6 h, parecoxib 40 mg 12/12 h, and bromopride 10 mg 8/8 h intravenous (IV). Morphine 2 mg IV on the night of surgery and 1 mg on postoperative day 1 (POD1) was maintained as a rescue medication, in addition to ondansetron 8 mg if necessary. Participants were assessed for pain using VNS (0, no pain; 10, unbearable pain), use of morphine as rescue analgesia, and presence of PONV during the stay in the PACU in the first 30 min of arrival, after 30 min, and at the time of discharge to the room. The same evaluation was performed the day after surgery. Furthermore, information was collected on the PACU's measurement of peripheral oxygen saturation (SpO2) and the need for supplemental O2.
Statistical analyses were performed using the R language (Version 3.6.3) RStudio software (version 2021.09.0 Build 351). Initially, a descriptive analysis of the data set was performed. Shapiro−Wilk normality and Bartlett’s homoscedasticity tests were applied. No parametric variables were found. Non-parametric variables were presented by median (interquartile range, IQR) and categorical variables by absolute number (percentage). The following methods were used to compare the groups: multivariate logistic regression for demographic data; Pearson’s chi-square test for categorical variables in which all groups had more than five counts; Fisher’s exact test for categorical variables in which at least one group had less than five counts; Wilcoxon’s test for non-parametric numerical variables. In all tests, a significance level of 5% was used.
3. RESULTS
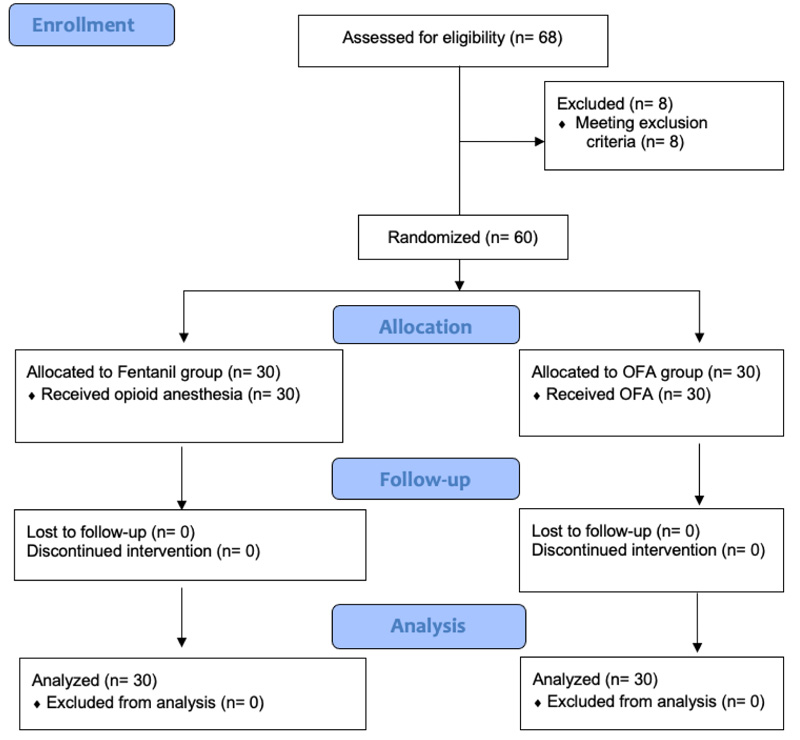
From January to August 2021, the study had 68 eligible patients. Of these, six were excluded because they were anesthetized by a professional who was not involved in the study and, therefore, did not follow the protocol. One participant was excluded for presenting with chronic pain, and one for presenting with quadriplegia. The 60 remaining participants were randomized into the groups “Fentanyl” (n = 30) and “OFA” (n = 30). There was no refusal to participate or loss to follow-up (Fig. 1).
The groups were comparable in age, gender, weight, height, BMI, physical status classification (ASA), surgical time, the volume of hydration, and presence or absence of smoking and hypertension (Table 2). One patient reported an allergy to dipyrone in the pre-anesthetic phase, and this medication was not used in this case. However, we decided to keep him in the study since dipyrone is considered a weak analgesic and would have little influence on the outcome.
| Moment of anesthesia | “Fentanyl” Group | “AOF” Group |
|---|---|---|
| Inductiona: | Fentanyl 2,5mcg.kg-1 | Magnesium sulfate 40mg.kg-1 Ketamine 25mg Lidocaine 1,5 a 2mg.kg-1 Dexmedetomidine 0,5mcg.kg-1 |
| Maintenancea: | Additions of Fentanyl 50mcg when necessary. Start saline solution 500mL 1mL.kg-1.h-1 |
Additions of Dexmedetomidine 0,25mg.kg-1 when necessary. Start analgesic solutionc 1mL.kg-1.h-1 |
| Conclusionb: | Sugammadex 2mg.kg-1 | Sugammadex 2mg.kg-1 |
bDoses based on corrected body weight (IBW +40%)
cAnalgesic solution according to text
| S.No | Variable | Fentanyl (30) | OFA (30) | P value |
|---|---|---|---|---|
| 1 | Gender (male/female) | 8 (26,7%) / 22 (73,3%) | 9 (30%) / 21 (70%) | 0,675 |
| 2 | Age (years) | 36 (9,3) | 35,7 (9,233) | 0,822 |
| 3 | Weight (kg) | 113,953 (18,958) | 116,17 (19,47) | 0,90 |
| 4 | Height (cm) | 1,679 (0,089) | 1,676 (0,096) | 0,941 |
| 5 | Body mass index (kg/m2) | 40,234 (4,272) | 41,163 (4,415) | 0,88 |
| 6 | ASA (II/III) | 17 (56,7%) / 13 (43,3%) | 13 (43,3%) / 17 (56,7%) | 0,418 |
| 7 | Smoking (yes/no) | 4 (13,3%) / 26 (86,7%) | 0 / 30 (100%) | 0,992 |
| 8 | Hypertension (yes/no) | 15 (50%) / 15 (50%) | 8 (26,7%) / 22 (73,3%) | 0,082 |
| 9 | Surgical time (min) | 148,333 (17,924) | 154 (27,241) | 0,396 |
| 10 | Hydration (mL) | 1407,333 (277,438) | 1418 (196,476) | 0,599 |
ASA, American Society of Anesthesiologists physical status.
The variable pain assessed by VNS did not show statistically significant differences at any of the moments analyzed. Morphine consumption (mg) was also comparable in both groups in the PACU and the ward. Four patients in the Fentanyl group and three in the OFA group had delayed discharge from PACU due to pain and did not show statistical differences (Table 3).
Likewise, the analysis of the adverse effects of opioids was similar between the groups. The presence of PONV, peripheral O2 desaturation (assessed as SpO2 < 92% in room air [RA]) and the need for supplemental O2 at discharge to the ward did not show statistical differences (Table 4). Patients who presented PONV in PACU (14 in the Fentanyl group, 9 in the OFA group) were treated with an additional dose of ondansetron 4 mg IV or promethazine 50 mg intramuscular (IM). All patients reported improved nausea after medication, and none presented vomiting during this evaluation. The day after surgery, one patient reported nausea and one other patient nausea and vomiting, which required rescue medication, both in the OFA group. Ten patients had SpO2 < 92% in RA at the time of arrival from PACU (2 in the Fentanyl group, 8 in the OFA group) and received 3−4 L/min of supplemental O2 mist (one of these patients used their own continuous positive airway pressure device – CPAP). Subsequently, all patients presented SpO2 > 95%, and those who did not tolerate the O2 mist discontinuation were discharged to the ward with a nasal catheter. All patients were discharged from the hospital on the first or second postoperative day, and none required intensive care unit (ICU) treatment. No severe adverse events were observed during the study. Episodes of hypotension were treated with ephedrine when necessary. No significant bradycardia (<50 bpm) occurred in any patient. In addition, although chronic pain was not an objective of this study, no patient presented pain at the return visit to the surgeon 3 months after the procedure.
| S.No | Variable | Fentanyl (30) | OFA (30) | P value |
|---|---|---|---|---|
| 1 | Pain at PACU (VNS) (mean of all values) |
5,333 (2,583) | 4,5 (2,333) | 0,289 |
| <30min | 7 (3,75) | 6 (2,75) | 0,449 | |
| >30 min | 5,5 (3) | 5 (3,5) | 0,275 | |
| Discharge | 3,5 (1,75) | 3 (2) | 0,703 | |
| 2 | Morphine consumption at PACU (mg) | 5 (3) | 4 (4) | 0,726 |
| 3 | Delay in discharge from PACU due to pain (yes/no) | 4 (13,3%) / 26 (86,7%) | 3 (10%) / 27 (90%) | 1 |
| 4 | Rescue morphine at night of the first day (2mg) (yes/no) | 6 (20%) / 24 (80%) | 6 (20%) / 24 (80%) | 1 |
| 5 | Pain (VNS) – POD1 (none/mild/moderate) | 8 (26,7%) / 20 (66,7%) / 2 (6,67%) | 2 (6,67%) / 25 (83,3%) / 3 (10%) | 0,127 |
| 6 | Rescue morphine (1mg) – POD1 (yes/no) | 0 / 30 (100%) | 1 (3,33%) / 29 (96,7%) | 1 |
PACU, post-anesthesia care unit; VNS, verbal numerical scale; POD1, postoperative day 1.
| S.No | Variable | Fentanyl (30) | OFA (30) | P value |
|---|---|---|---|---|
| 1 | PONV at PACU (yes/no) | 14 (46,7%) / 16 (53,3%) | 9 (30%) / 21 (70%) | 0,288 |
| 2 | PONV at POD1 (yes/no) | 0 / 30 (100%) | 2 (6,67%) / 28 (93,3%) | 0,492 |
| 3 | SpO2 RA <92% when arriving PACU (yes/no) | 2 (6,67%) / 28 (93,3%) | 8 (26,7%) / 22 (73,3%) | 0,08 |
| 4 | Desaturation (SpO2 RA <92%) during PACU (yes/no) | 5 (16,7%) / 25 (83,3%) | 11 (36,7%) / 19 (63,3%) | 0,144 |
| 5 | Discharge from PACU with supplemental oxygen (yes/no) | 1 (3,33%) / 29 (96,7%) | 5 (16,7%) / 25 (83,3%) | 0,195 |
4. DISCUSSION
Laparoscopic gastroplasty analgesia has always been considered difficult to manage postoperatively. Considering the increasing number of publications on opioid-sparing and opioid-free anesthesia, with several studies demonstrating the advantages of this technique [1, 12, 13], the OFA protocol proposed in this study was created to optimize the care of these patients.
This clinical study did not show significant differences in pain or the use of rescue morphine postoperatively. Similarly, the rate of PONV and peripheral oxygen desaturation was not statistically different between the groups.
Few studies with OFA have been performed in bariatric surgery. Several clinical trials studied the analgesic and opioid-sparing effect of adjuvant drugs such as ketamine, lidocaine, magnesium sulfate, and dexmedetomidine but maintained anesthesia with fentanyl or remifentanil, making comparison difficult [14-16]. The literature shows that there is still no consensus on which drugs to use or which doses, and each service adopts its own protocol [17-19].
In the study by Ziemann-Gimmel [20], which included 119 patients, anesthesia with fentanyl versus OFA was compared, and no differences in pain or postoperative opioid consumption were shown. However, a reduction in PONV rate was observed, but the comparison was made between a group with inhalation anesthesia and another with total venous anesthesia.
A study by Tufanogullari [21] with 80 patients showed that dexmedetomidine in gastroplasty was associated with a lower PONV, but the same group also used less rescue fentanyl after awakening from anesthesia. Pain scores and quality of recovery were not different between the two groups.
A recent study by Mulier [22] compared 45 laparoscopic gastroplasty patients with sufentanil versus OFA. A lower PONV was observed in the OFA group, but this group was associated with the use of a lower dose of morphine in PACU (mean, 4.9 mg × 15.3 mg). Pain evaluated by the visual analog scale was also lower in this group. However, the rescue dose of morphine required in the opioid anesthesia group was 15.3 mg, well above that used in the “Fentanyl” group in our study (5 mg). The study by Mulier also found a higher desaturation rate in the group with sufentanil. However, episodes of hypoxemia were observed even with the use of a 6 L/min O2 mask. Our study evaluated hypoxemia in RA, and no patient had SpO2 < 95% with supplemental O2.
A recent systematic review with meta-analysis [23] of 33 clinical trials evaluating OFA versus opioid anesthesia in various types of surgery did not show a difference in pain scores or postoperative use of morphine, corroborating the results found in our study. However, PONV rates were significantly lower in the group without opioids. We do not know whether this better outcome with nausea and vomiting may have been influenced by the protocols used, as most of them used dexmedetomidine in continuous infusion. The largest research included in this review was a multicenter study that compared remifentanil versus OFA with dexmedetomidine in non-cardiac surgery [24]. Although morphine consumption and PONV were lower in the OFA group, this group had more episodes of hypoxemia and adverse events and was discontinued due to five cases of severe bradycardia. The dose of dexmedetomidine was higher than in our study and was administered in continuous infusion.
This study had limitations, and some factors could have influenced our outcomes. First of all, it was not possible to standardize the use of the bispectral index (BIS) and train four (TOF) monitors. Due to the difficulty in obtaining the device for all participants, we chose not to use it in any of them. Studies showed that dexmedetomidine or drugs, such as lidocaine, ketamine, or clonidine, reduced the need for inhalation anesthetics [15, 21, 25]. However, as we couldn’t use BIS in this study, a standard dose of sevoflurane was used in all patients (0.9−1 MAC), and we may have lost possible benefits with a lower MAC in the OFA group, especially regarding PONV [26]. The non-use of TOF also meant that rocuronium was standardized with intraoperative doses every 40 min of surgery. Therefore, it is not possible to say about the quality of muscle relaxation and whether this may have interfered with the result. Some studies associated better relaxation with lower intraabdominal pressure during the pneumoperitoneum and less postoperative pain [27]. In addition, neither the level of sedation nor the patient’s comfort or satisfaction was assessed, and analgesia was evaluated by VNS, even when the patient did not complain of pain. It’s known that psychological and psychiatric illnesses are more common in obese patients than in the general population [28], and no preoperative tests assessing these problems were made. Therefore, we cannot guarantee that those were distributed equally in both groups. Besides, differently of most OFA protocols in literature, we chose clonidine in continuous infusion instead of dexmedetomidine. Both drugs have already been associated with decreased postoperative pain and opioid consumption [29], but the dose used in our study could be lower than it’s required to see an opioid-sparing effect [30].
Although we could not demonstrate a benefit of OFA compared to opioid anesthesia, our protocol was effective and safe. In a world looking for alternatives to reduce opioid use and abuse, this method is a feasible option and deserves physicians’ attention. There are several OFA protocols, and each experience with these drugs should be shared. More studies on this subject would help to clarify and optimize this modality of anesthesia.
CONCLUSION
The present study did not find benefits of OFA compared to general anesthesia with opioids. However, this protocol was shown to be safe and effective.
LIST OF ABBREVIATIONS
| OFA | = Opioid-free Anesthesia |
| PONV | = Postoperative Nausea and Vomiting |
| VNS | = Verbal Numerical Scale |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was submitted for review and approval by the Research Ethics Committee of the Health Sciences Sector of the Federal University of Paraná (CEP/SD), CAAE 39513520.3.0000.0102 (plataformabrasil.saude.gov.br; approval date 07/12/2020).
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans used were in accordance with the Research Ethics Committee of the Health Sciences Sector of the Federal University of Paraná and with the Helsinki Declaration of 1975.
CONSENT FOR PUBLICATION
Informed consent has been obtained from the participants involved.
STANDARDS OF REPORTING
CONSORT guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data supporting this study's findings are available within the article.
FUNDING
Dr. Juliana Thomaz Menck has received a Scholarship from the Surgical Department of The Federal University of Parana (CAPES – DS agency, a government program, by the number 40001016018P0). For the remaining authors, none were declared.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise
ACKNOWLEDGEMENTS
Declared none.


