All published articles of this journal are available on ScienceDirect.
Oral Ergotamine versus Theophylline as Treatment of Post-dural Puncture Headache (PDPH) in Cesarean Section: A Randomized Clinical Trial
Abstract
Introduction:
Post Dural Puncture Headache is (PDPH) a relatively common complication of spinal anesthesia. This study aimed to compare the effect of oral administration of ergotamine and theophylline on PDPH in patients undergoing a cesarean section.
Materials and Methods:
This clinical trial was performed on 60 parturients undergoing cesarean section with PDPH. A tablet of theophylline (100 mg) or methyl ergotamine C (1 mg plus 100 mg caffeine) every 8 hours for 24 hours was administered randomly to patients referred to the hospital with PDPH. Using a checklist, demographic information, history of previous PDPH, number of punctures and intensity, location, and onset time of headache were collected. Intensity and duration of PDPH in the first 24 hours after surgery were the primary outcomes and nausea, vomiting, and vertigo were considered secondary outcomes. The intensity of the headache was assessed using Visual Analog Scale (VAS) before and within the first 24 hours after drug administration.
Results and Discussion:
In both groups, the VAS of headache significantly decreased at 2, 8, and 24 hours after administration of theophylline and ergotamine compared to pre-intervention time (theophylline from 8.6 ± 1.1 to 0.2 ± 0.1 and ergotamine from 8.6 ± 1.5 to 0.4 ± 0.2). However, the intensity of headaches was not different between the two groups at 2, 8, and 24 hours after the intervention. Duration of headache was similar in both groups (15.7 ± 5.9 in the theophylline group versus 17.5 ± 14.2 ergotamine group). In terms of secondary outcomes of nausea, vomiting, and vertigo, both groups were comparable.
Conclusion:
Oral administration of theophylline and ergotamine are similarly effective in reducing PDPH in cesarean sections.
Clinical Trial Registration Number:
IRCT20120915010841N14.
1. INTRODUCTION
Post-dural puncture headache (PDPH) is one of the complications of spinal anesthesia that occurs in 3-17.9% of patients [1]. Loss of cerebrospinal fluid, decrease in intracranial pressure, and subsequent compensatory vasodilation are the possible causes [1]. Increasing the diameter of the needle used in spinal anesthesia, more punctures for spinal anesthesia, a previous history of post-spinal anesthesia, and a history of chronic headaches are associated with a higher incidence of headaches [2]. Headaches can be severe enough to disrupt a person's life, and may even last days and weeks, being uncomfortable for a mother who has recently had a child and may have difficulty with breastfeeding and infant care [3].
Early treatment of headaches is symptomatic and supportive, including rest, fluid administration, medication, and eventually the Epidural Blood Patch (EBP) [1]. Studies have shown that drugs, such as caffeine, aminophylline, pregabalin, gabapentin, somatotropin, methergine (methylergonovine), dexamethasone, and hydrocortisone, are effective in the treatment of PDPH [4]. However, in this regard, there is still no general agreement [5-7]. The ergotamine C tablets cause cerebral vasoconstriction through stimulation of alpha-adrenergic receptors and norepinephrine reuptake inhibition. They are used for the treatment of vascular headaches (migraine and clustering). Given this mechanism, they appear to be effective in the treatment of PDPH. Also, the caffeine contained in this pill is also used in the treatment of PDPH due to its vasoconstrictor effects on the brain vessels; it increases the rate of ergotamine uptake [8].
Theophylline tablets, which are among methyl xanthines, cause cerebral vasoconstriction through inhibition of phosphodiesterase and increase in cellular CAMP concentration and antagonistic effects of adenosine receptors and can be used to treat PDPH [9]. If drugs are not effective, invasive methods, such as Epidural Blood Patch or Sphenopalatine Ganglion Block (SPGB) [10], are used, which are less well-known due to their invasive and associated complications. Various studies have investigated the efficacy of injecting caffeine, theophylline, and aminophylline intravenously in the prevention and treatment of PDPH, with different results reported. Most studies have focused on the effect of theophylline or aminophylline in PDPH treatment with placebo; however, so far, no studies have compared the effects of theophylline and ergotamine on PDPH treatment. Therefore, we decided to evaluate and compare the effect of oral administration of theophylline and ergotamine C in PDPH treatment.
2. MATERIALS AND METHODS
This randomized clinical trial was conducted with the permission of the Ethical Committee of Hamadan University of Medical Sciences (IR.UMSHA.REC.1397.415) and registered at the Clinical Trials Center by the code IRCT20120915010841N14 at Fatemieh Hospital in Hamedan in 2018. Data collection tools included a researcher-made checklist tailored to the purpose and variables of the study to record the scores of pain, drug side effects, and patient satisfaction. Consecutive sampling was performed on women undergoing cesarean section with spinal anesthesia who had PDPH and met the inclusion criteria. Sixty subjects were selected.
2.1. Inclusion Criteria
Pregnant women of 18 to 45 years old with ASA of class I and II undergoing cesarean section with spinal anesthesia and having PDPH referred to the hospital without any disease of heart, liver, kidney, thyroid, diabetes, gastric ulcer, seizures, allergies to the drug, Raynaud's disease, hypocalcemia and a history of chronic headaches (migraine and sinusitis).
2.2. Exclusion Criteria
Patients who did not establish communication and answer questions. Patients who had drug side effects and did not continue treatment after taking the drugs were excluded from the study.
After providing sufficient explanations and obtaining informed consent from patients undergoing cesarean section with spinal anesthesia and having PDPH that referred to Fatemieh Hospital for treatment, 60 patients were selected and randomly placed in four blocks, and one of two groups of theophylline (A) and ergotamine (B). Patients were first asked about the number of punctures, previous history of PDPH, time of onset and location of headache, and initial VAS of headache. The VAS (Visual Analogue Scale of Pain) of headache was recorded using a 10 cm ruler (zero to ten degrees). The patient marked it based on the severity of the pain and the number indicated on the ruler showed the severity of the patient’s pain.
In group A, a theophylline tablet (100 mg) and in group B, an ergotamine C tablet (containing 1 mg Methyl Ergonovine and 100 mg caffeine) every 8 hours were prescribed for 24 hours by an anesthesiologist with adequate explanations on how to administer the drugs. Considering that our patients were breastfeeding women, we decided to evaluate the minimum dose (5 mg/kg) of theophylline for the treatment of PDPH (theophylline dose is 5-10 mg/kg in divided doses). Also, the patients were not aware of the type of medication prescribed. Ergotamine and theophylline were given to patients within the first 24 hours and patients were told to avoid breastfeeding during this period.
However, it should be noted that hydration and analgesics (acetaminophen 500 mg every 6 hours in case of VAS pain of more than 4) were also administered to patients in both groups. Then, 24 hours after drug administration, patients were contacted by a researcher unaware of the drug prescribed to them by telephone and asked them about VAS pain, the amount of acetaminophen consumed, complications, such as nausea and vomiting, hot flashes, tinnitus, dizziness and epigastric pain, and satisfaction level. The results were recorded in a questionnaire.
In the current study, software SPSS version 16 was used for data analysis. Statistical significance was considered below 5%. Data description was performed using descriptive statistics with mean and standard deviation for quantitative variables and ratio and percentage for qualitative variables. To compare the relationship between qualitative variables with each other, the Chi-square test and that of quantitative variables, t-student or its parametric equivalent were used. The sample size was calculated based on the findings of the study by Mahoori et al. [11] with a power of 80% and a type 1 error of 0.05 by using G*Power software.
3. RESULTS
In this study, 60 parturients, who underwent cesarean section with spinal anesthesia, had PDPH, and met the inclusion criteria, were randomly assigned into two groups of 30 parturients receiving either theophylline or ergotamine C (Fig. 1). A total of 19 patients in group A and 18 patients in group B had PDPH on the first day after cesarean section.
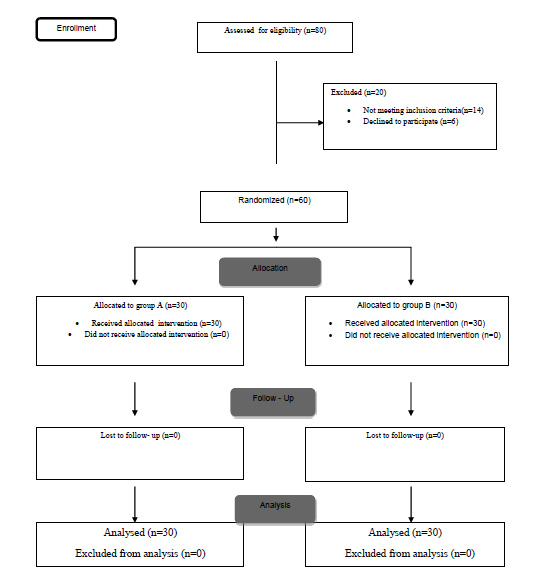
The mean age of patients was 28.8 ± 6.6 in the theophylline group and 30.1 ± 7.1 in the ergotamine C group (P = 0.465). Mean initial headache severity (VAS) was 8.6 ± 1.1 in the theophylline group and 8.6 ± 1.5 in the ergotamine C group (P = 1.00). The mean time to onset of headache was 30.2 ± 20.8 in the theophylline group and 40.5 ± 20.1 in the ergotamine C group (P = 0.154). A total of 3.3% of the theophylline group and 10% of the ergotamine group had previous PDPH (P = 0.612).
The number of punctures more than once was 60% in the theophylline group and 73.3% in the ergotamine group (P = 0.545).
In both groups, the intensity of headache decreased at 8 and 24 hours after administration of theophylline and ergotamine. There was a statistically significant reduction compared to pre-intervention (Theophylline from 8.6 ± 1.1 to 0.2 ± 0.1 and Ergotamine from 8.6 ± 1.5 to 0.4 ± 0.2). However, the difference between the two groups in headache intensity before intervention and at 2, 8, and 24 hours after the intervention was not significant. This means that the decrease in VAS of headaches was similar in the two groups. Duration of headache after the intervention was similar in both groups and was between 15 and 18 hours (15.7 ± 5.9 in the theophylline group versus 17.5 ± 14.2 in the ergotamine c group) (Table 1).
| Variable | Group A mean ± Standard deviation |
Group B mean ± Standard deviation |
P (t-test) |
|---|---|---|---|
| After spinal | 8.6 ± 1.1 | 8.6 ± 1.5 | 1.00 |
| 2 hours after the intervention | 6.4 ± 1.2 | 6.7 ± 1.9 | 0.379 |
| 8 hours after the intervention | 2.4 ± 1.6 | 3.5 ± 2.5 | 0.061 |
| 24 hours after the intervention | 0.2 ± 0.1 | 0.4 ± 0.2 | 0.269 |
| Duration of headache (hour) | 15.7 ± 5.9 | 17.5 ± 14.2 | 0.506 |
There was no significant difference in the frequency of vertigo, tinnitus, epigastric pain, hot flashes, nausea, and vomiting in both groups. Nausea and vomiting were the most frequent complications. None of the patients had tinnitus, epigastric pain, and hot flash (Table 2).
| Variable | Group A Frequency (percentage) |
Group A Frequency (percentage) |
P (chi2) | |
|---|---|---|---|---|
| Nausea and vomiting | Yes | (16.7) 5 | 3 (10.0) | 0.448 |
| No | (83.3) 25 | 27 (90.0) | ||
| Vertigo | Yes | 1(3.3) | - | 0.313 |
| No | 29 (96.7) | 30 (100.0) | ||
Patients' satisfaction and analgesic use in the ergotamine and theophylline groups were not significant (P = 0.063), indicating that the two groups had similar analgesic consumption and satisfaction. The most common site of headache was frontal (33.8%) and 8.1% of patients had diffuse pain. A total of 18.9% of patients had neck stiffness, which was similar in both groups.
4. DISCUSSION
The findings of the present study showed that oral administration of 100 mg of theophylline and 1 mg of ergotamine every 8 hours in patients undergoing cesarean section with spinal anesthesia who was suffering from PDPH demonstrated good effects on the relief of headaches. Both drugs had no significant adverse effects, such as nausea and vomiting. In a similar study conducted by Wu and colleagues [12], in which patients with PDPH were administered 250mg of intravenous aminophylline, it was observed that VAS decreased from 7.72 at 30 and 60 minutes to 4.8 + 2.53 and 3.56 + 2.06 and 1.44 + 1.87 on the first and second days, respectively. They concluded that intravenous administration of aminophylline is effective and safe in the treatment of headaches after spinal anesthesia. In a similar study conducted by Ergün colleagues [13], the effects of theophylline and placebo were compared in the treatment of PDPH. It was concluded that the VAS of headache decreased from 7.05 + -1.47 (before theophylline administration) to 2.88 + -2.31 (after theophylline injection), which was consistent with the findings of the present study.
In the study of Yang et al. [14], it was reported that prophylactic administration of 250 mg of aminophylline 30 minutes after the cesarean section was associated with a reduced incidence of PDPH compared to the placebo (3.4 vs. 17.2%), which also had not serious complications in patients.
Sadeghi and colleagues [15] demonstrated that intravenous infusion of aminophylline 1.5 mg/kg after the birth of neonates in women who underwent cesarean section with spinal anesthesia decreased the risk of PDPH at 24 and 48 hours after surgery with a significant difference from the placebo group. As can be seen in the above studies and our study, aminophylline administration was effective in reducing the incidence of PDPH. However, in a study conducted by Zajac and colleagues [16], it was found that oral administration of caffeine and intravenous administration of magnesium (2 gr) and aminophylline (250 mg) daily had no effect on reducing the incidence of PDPH in patients who underwent cesarean section with spinal anesthesia, which was inconsistent with the results of the present study. In a study carried out by Schwalbe and colleagues [17], theophylline was found to be effective in the treatment of PDPH; it was consistent with the results of the present study.
There are few studies on the use of ergotamine in the prevention or treatment of PDPH; therefore, further studies are needed. In a study conducted by Hakim and colleagues [18], the intravenous administration of methyl ergonovine was found to be effective in treating PDPH due to cesarean section under spinal anesthesia, which was consistent with the results of the present study. The results of a study conducted by Saper and colleagues showed that oral administration of methyl ergonovine was effective in the prevention of refractory migraine headaches [19]. In a study, Blaha and colleagues [20] reported that caffeine administration reduces cerebral blood flow and can be used to treat headaches associated with cerebral vasodilation, which is consistent with the results of the present study. Methyl ergonovine maleate is a useful drug for controlling postpartum hemorrhage and is effective in reducing uterine bleeding by inducing uterine contractions. On the other hand, it activates the vomiting center in the medulla and serotonin receptors in the gastrointestinal tract and may cause nausea and vomiting. The results of the present study also showed that about 10% of patients complained of nausea and vomiting. Overall, as confirmed by other studies, it can be concluded that since methyl ergonovine is effective in controlling postpartum hemorrhage, it can be a plausible choice for the relief of headaches in patients undergoing cesarean section with spinal anesthesia. The results of a study conducted by Anvari Pour et al. [21] demonstrated that cesarean patients who received methyl ergonovine had less need for vasoconstrictors and more stable hemodynamic conditions.
Since theophylline and ergotamine are easy to use and their side effects are less, they are easily accepted by the patient and can be a good alternative to more aggressive methods, such as an epidural blood patch or intravenous drug injection. The relatively small sample size and the measurement of pain intensity based on the person's opinion (subjective) were the limitations of the present study.
CONCLUSION
Oral administration of theophylline and ergotamine C are both effective in the treatment of post-dural puncture headaches in cesarean patients and have similar efficacy.
LIST OF ABBREVIATIONS
| PDPH | = Post-dural Puncture Headache |
| VAS | = Visual Analog Scale |
| EBP | = Epidural Blood Patch |
| SPGB | = Sphenopalatine Ganglion Block |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This randomized clinical trial was conducted with the permission of the Ethical Committee of Hamadan University of Medical Sciences (IR.UMSHA.REC.1397.415).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
STANDARDS OF REPORTING
CONSORT guidelines were followed in the study.
FUNDING
This work was supported by Hamadan University of Medical Sciences (No: 980120136).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


