All published articles of this journal are available on ScienceDirect.
The Effect of Preoperative Gabapentin on the Duration of Spinal Anaesthesia in Patients Undergoing Lower Limb Surgery: A Randomized Controlled Clinical Trial
Abstract
Background:
In the present study, we aim to examine oral gabapentin efficacy as an adjuvant to spinal anesthesia.
Methods:
This prospective, clinical, randomized trial included subjects between 20 and 60 years undergoing lower limb surgeries categorized into two cohorts. Group (G) received gabapentin (900 mg) in two divided doses prior to spinal anesthesia; (300 mg 10 hrs prior to spinal anesthesia induction as well as 600 mg 2 hrs before spinal anesthesia). In contrast, the control Group (C) received only spinal anesthesia. The onset, as well as the duration of spinal anesthesia, were the primary outcome, while the secondary outcome was the postoperative nalbuphine consumed.
Results:
A total of 60 cases were evenly categorized into two cohorts. Both groups demonstrated no differences regarding motor and sensory block onset and duration. The group receiving preoperative gabapentin had a significant decrease in postoperative nalbuphine consumption with a mean of 20.8±9.4 mg compared to the control group, which showed an increased consumption of 28.9±10.4 mg with a p-value of 0.006. Further analysis of the Visual Analog Score (VAS) in both groups revealed that the decrease in total nalbuphine consumption was found at (8 and 10 hrs) postoperatively, with p values of 0.016 and 0009, respectively.
Conclusion:
Gabapentin administration (900 mg) within 10 hrs of surgery in two subdivided doses prior to spinal anesthesia had no effect on onset and duration of spinal anesthesia but had a delayed beneficial postoperative analgesic effect.
Clinical Trial Registration Number:
This trial was registered at ClinicalTrials.gov (NCT05659810, URL: https://clinicaltrials.gov/ct2/show/NCT05659810in 21st December 2022.
1. INTRODUCTION
Spinal anesthesia is a form of neuraxial anesthesia that is an efficient and secure substitute for general anesthesia in surgeries of the lower extremity, perineum, as well as lower abdominal. Spinal anesthesia has numerous merits compared to general anesthesia, including shorter post-anesthesia care unit stay, decreased postoperative vomiting and nausea, improved pain management, as well as less blood loss [1, 2]. However, the anesthesiologist faces numerous obstacles during spinal anesthesia, such as limited duration, hemodynamic instability, delayed recovery of motor function, and headache [3].
Several adjuvant drugs have been intrathecally utilized for prolonging spinal anesthesia durations, in addition to decreasing the dose of the local anesthetic. Consequently, they decrease side effects like hypotension and bradycardia. Opioids such as morphine, fentanyl, midazolam, clonidine, dexmedetomidine, and dexamethasone are among the drugs used [4-9].
Gabapentin, a 1994-approved anticonvulsant, is a structural analog of the inhibitory neurotransmitter gamma-aminobutyric acid. Anticonvulsants such as carbamazepine have been utilized for treating certain varieties of neuropathic pain for decades [10].
A number of studies have demonstrated gabapentin efficacy in minimizing pain scores and elevating analgesia mean duration when utilized for surgeries performed under spinal anesthesia, such as total abdominal hysterectomy, orthopedic surgeries, and surgeries on the spine [11-14].
The study hypothesized that a gabapentin preoperative dose (900 mg) administered in two doses might increase spinal anesthesia duration in cases undergoing surgeries in lower limbs.
2. MATERIALS AND METHODS
2.1. Study Design
After Institutional Review Board approval (R 177/2022), this trial was registered at ClinicalTrials.gov (NCT05659810, URL: https://clinicaltrials.gov/ct2/show/NCT05659810in 21st December 2022. Prior to enrollment, all subjects signed informed consent.
The study was conducted at our hospital from December 2022 to April 2023. Following admission to our hospital for surgery, our anesthesia residents recruited patients from the Anesthesia Clinic. Patients were randomly assigned using computer-generated block randomization developed by the first generator of randomization.com. After generating number tables, they were placed in opaque sealed envelopes by an anesthesiologist who did not participate in the study.
Adult patients of both sexes who had lower limb fractures based on the American Society of Anaesthesiologists I-III, aged 18-65 years, and weighing between 40 and 80 kg undergoing elective lower limb surgery, were included in this study. Patients with coagulopathies, spinal anesthesia contraindications, and elevated liver enzyme levels were excluded from our study.
Eligible patients were randomly assigned to two equal groups (each containing 30). Subsequently, Group (G) received gabapentin (900 mg) prior to spinal anesthesia [15]. In contrast, Group (C) received only spinal anesthesia (control group).
2.2. Study Procedures
On the day prior to surgery, all subjects received a standard preoperative assessment (clinical examination, history taking, and routine laboratory investigations). Group (G) received gabapentin (900 mg) in two divided doses prior to spinal anesthesia, as mentioned previously, while Group (C) received none.
On admission to the operating room (OR), recording heart rate as well as blood pressure was done, an intravenous catheter was inserted under aseptic conditions, and 500ml of Ringer solution was administrated. Preoperative vital data, including heart rate, blood pressure, and SpO2, were collected. The patient’s vital signs were monitored until PACU discharge.
Patients were seated following sterilizing their back with povidone-iodine local infiltration using 3 ml lignocaine at the needle entry site. A 3.0 ml-midline intrathecal injection of 0.5% hyperbaric bupivacaine at L 3/4 or L4/5 was performed utilizing a 25G needle.
Assessment of neural block was done via the pinprick test (Hollman test) 3-point scale; (0) indicates normal sensation, (1) indicates pinprick sensation loss (analgesia), and (2) denotes touch sensation loss (anesthesia) [16]. The assessment of the motor block was done utilizing a modified Bromage scale (Bromage 0); the patient is able to move ankles, knees, and hips. Bromage 1 is when he is able to move the ankle and knee but not the hips. Bromage 2 is when he can move the ankle, not the knees or hips. Bromage 3 when he cannot move ankles, knees, and hips [17]. Recording results was done every 3 min until reaching a stable level for three consecutive tests.
Following a successful intrathecal injection, subjects were monitored continuously for block progression as well as complications. Afterward, the patient’s blood pressure was measured (every 3 min and when needed). The following were monitored in the patient:
Block progression: The block should be adequate for the surgical procedures and should not progress excessively.
Hypotension definition was set as a decline in MAP>20% of the patient’s basal MAP and was changed due to an intravenous (IV) bolus of 250-500 ml Ringer solution, and if no response was recorded, an IV bolus of ephedrine 5 - 10 mg was given.
Bradycardia definition was set as a decline in HR<60 beats/min and was treated by an IV bolus of atropine 0.02 mg/kg.
Patients were continually monitored until the conclusion of the surgery before transfer to PACU, then the ward.
2.3. Data Measurement
2.3.1. The following Data were Recorded During the Trial
- Demographics as well as characteristics of patients (body weight, age, body mass index (BMI), Intraoperative hemodynamics (including mean heart rate as well as blood pressure in timing as indicated below):
(T0: preoperatively as baseline; T1: following spinal anesthesia administration; T2: at skin incision, then every 15 min until the surgery ends.
- Assessment of onset and time of regression (duration) of Sensory Block:
Assessment of sensory block was done utilizing the pinprick test (Hollman test).
The sensory block onset time was defined as the time between the local anesthetic administration and the complete sensory block (score 2 for all nerves). The sensory block duration was set as the interval between a complete sensory block and anesthesia resolution (score 0 for all nerves).
- Assessment of motor block’s onset and regression time (duration):
- Utilizing a modified Bromage scale, the motor block was determined. The time between the injection as well as block completion was deemed as motor block onset. Motor block duration was defined as the interval between full motor block and anesthesia resolution. The block was deemed unsuccessful when sensory and motor blocks did not occur until 20 min.
- Pain assessment: subjects were monitored every 2 hrs for analgesic requirements for the first 12 hrs following surgery. Evaluation of postoperative pain was done utilizing the visual analog scale (VAS) [18], and in case the VAS >4, the patient received 0.25 mg/kg nalbuphine.
2.4. Outcomes
The primary outcome was the duration of spinal anesthesia determined by the sensory block pinprick test, besides the motor block Bromage scale. The secondary outcome was the total opioid consumption for postoperative pain management, guided by the VAS assessed every 2 hours for 12 hours postoperatively.
2.5. Sample Size Calculation
Calculation of sample size was done utilizing the 11th version of NCSS PASS as per Gogna et al., 2017 [19]. A total of 60 patients, 30 in each group, are required to obtain 99% power to detect a difference of 70,1 between the null hypothesis that the means of both groups are 288.8. The alternative hypothesis is that the mean of Group C is 218.7, with estimated group standard deviations of 38.8 and 37.6 and a significance level (alpha) of 0.05 based on a two-sided two-sample t-test. The primary outcome is the duration of spinal anesthesia. The sample size was inflated by 20.0% to accommodate for the prospective studies’ attrition problem.
2.6. Statistical Analysis
The expression of results was in the form of mean ± standard deviation or number (%). In addition, a comparison of categorical data was made utilizing the chi-square test, whereas the unpaired t-test was utilized for different numerical data in both groups. Comparison between different times of measurements and baseline within the same group was performed using repeated-measures analysis of variance. SPSS for Windows version 16.0. (SPSS Inc.-Chicago-IL-USA) was utilized for analyzing data. P-values <0.05 were deemed significant.
3. RESULTS
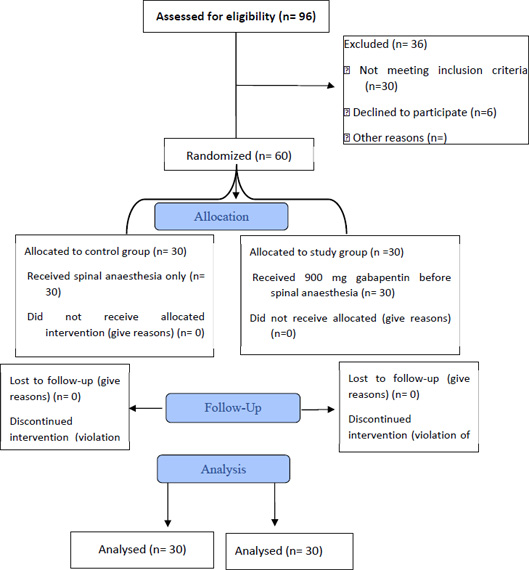
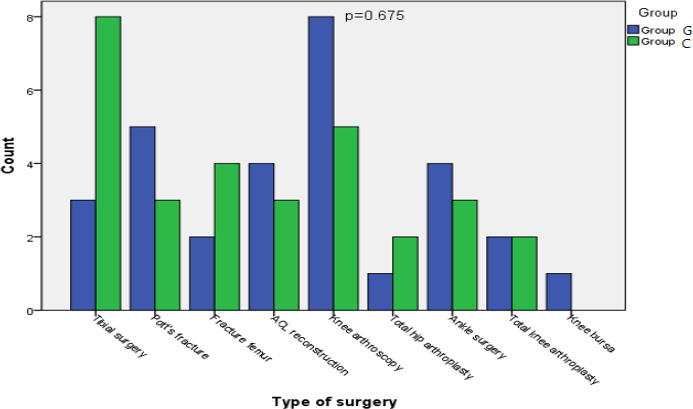
Ninety-six patients scheduled for lower limb surgeries were assessed for eligibility to participate in our study 6 declined to participate, and 30 patients were excluded for not meeting all our inclusion criteria. We obtained informed consent from 60 patients randomized into either the control or interventional groups, as shown in Fig. (1). All patients were analyzed, and none were lost in follow-up. Statistical analysis showed no difference regarding age, sex, ASA classification, and BMI between both groups (Table 1). No discernible differences were detected between both groups regarding the type of surgery (Fig. 2).
As regards the vital intraoperative data, including mean pressure of arterial blood as well as heart rate, no difference was detected between the two groups, whether before or after inducing the spinal anesthesia (Table 2).
No difference was detected regarding the characteristics of the block, including either the sensory or motor block onset or duration between the two groups (Table 3).
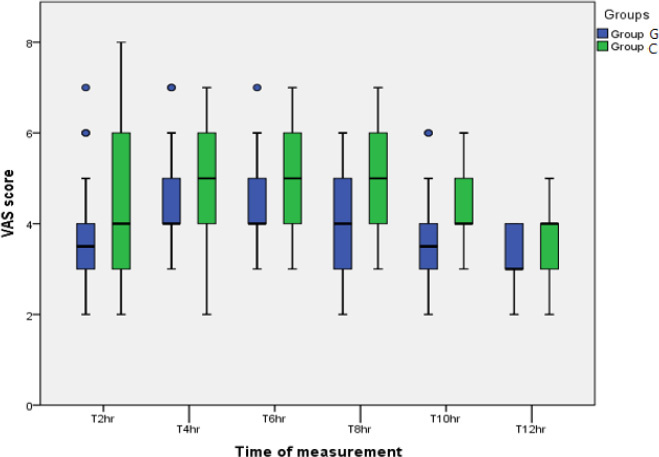
For postoperative analgesia, the VAS score was reduced in Group G than in Group C, especially at 8 and 10 hrs postoperatively, but not 2, 4. 6, and 12 hrs (Table 4) (Fig. 3). Opioid consumption declined in Group G than in Group C (Table 3).
4. DISCUSSION
In this study, we investigated oral gabapentin’n effect on the onset of spinal anesthesia duration, as well as its impact on postoperative analgesia. Gabapentin is absorbed slowly after oral administration reaching peak plasma concentration after 3-4 hours, and maximum bioavailability at a dose of 900mg dropping from 60% to 33% as the dose increase to 3600mg [15]. So we chose the dose of 900mg; however, we failed to demonstrate any impact of oral gabapentin on spinal anesthesia onset and duration, but we did find a clinically significant, albeit delayed, difference in postoperative analgesia.
Numerous studies have examined oral gabapentinamined inion the postoperative period.
Elazzazi H et al., illustrated that preoperative administration of 1500 mg gabapentin reduced pain severity for up to 24 h [12]. Schmidt and his colleagues concluded that elevated preoperative gabapentin dosage (1200 mg) is more effective than lower doses [20]. The pre-emptive administration of gabapentin approximately 2 h before surgery appears effective in achieving maximal plasma concentrations during surgical stimuli [11].
An earlier study performed by Gogna et al., in 2017 examined the efficacy of a single preemptive dose of gabapentin (600 mg) on the duration of postoperative analgesia in cases undergoing spinal anesthesia surgeries. They found that patients receiving the drug had better postoperative pain control at all time points compared to the placebo group [19].
Verma and his colleagues also demonstrated that the administration of 300 mg of gabapentin 2 h before surgery substantially reduced pain and epidural boluses requirement in cases undergoing total abdominal hysterectomy [21].
| - | Group G(n=30) | Group C(n=30) | P-value |
|---|---|---|---|
| Age (years)Sex (M/F)ASA (I/II)BMI | 38.2 ± 12.0420/1018/1227.5 ± 3.98 | 39.2 ± 11.117/1319/1127.9 ± 3.3 | 0.731#0.426ο0.791ο0.701# |
| - | Group G(n=30) | Group C(n=30) | P-value |
|---|---|---|---|
| HR0 (b/m)(baseline)HR1(after spinal anesthesia)HR2(at skin incision)MBP0(mmHg)(baseline)MBP1(after spinal anesthesia)MBP2(at skin incision) | 79.6 ± 10.8466.7 ± 10.4570.8 ± 17.3397.3 ± 7.5283.6 ± 9.8681.1 ± 9.43 | 82.5 ± 12.870.9 ± 14.484.1 ± 24.999.9 ± 8.382.5 ± 10.977.8 ± 8.4 | 0.3530.2020.0210.2080.6830.167 |
| - | Group G(n=30) | Group C(n=30) | P-value |
|---|---|---|---|
| The onset of sensory block (min)Duration of sensory block (min)The onset of motor block (min)Duration of motor block (min)Total opioid dose(mg) | 7.3 ± 1.44191.7 ± 32.397.1 ± 1.24146.2 ± 25.7620.8 ± 9.49 | 7.5 ± 1.3186.6 ± 21.27.3 ± 1.2137.5 ± 21.828.9 ± 10.5 | 0.5140.4690.5300.1650.006* |
| - | Group GN=30 | Group CN=30 | P-value |
|---|---|---|---|
| VAS after 2hrsVAS after 4hrsVAS after 6hrsVAS after 8hrsVAS after10hrsVAS after12hrs | 3.5 (2-7)4 (3-7)4 (3-7)4 (2-6)3.5 (2-6)3 (2-4) | 4 (2-8)5 (2-7)5 (3-7)5 (3-7)4 (3-6)4 (2-5) | 0.3470.3740.1550.013*0.009*0.031 |
We tested higher gabapentin doses in our study compared to the studies by Gogna et al. and Anil Verma et al. However, we did not find that the postoperative pain scores demonstrated by the VAS score were lower than the control group postoperatively through all points of time, as stated by Gogna et al. However, it decreased between 8-12 hours postoperatively. This result can be attributed to the synergistic effect of gabapentin when administered with an opioid analgesic, which our patients received when they experienced pain [22].
In contrast, the meta-analysis published in anaesthesiology by Verret et al. in 2020, which included 281 clinical trials, revealed a relatively diminished postoperative pain intensity at 6, 12, 24, and 48 h when gabapentinoids are administrated. This difference was not clinically significant, aligning with our findings [23].
Numerous studies, including the 2016 study by MiHye Park and Younghoon Jeonin [24] and the 2020 study by El-Kady et al. [25], have demonstrated that pregabalin (a gabapentinoids similar to gabapentin) significantly increases spinal anesthesia duration. Therefore, we hypothesized that gabapentin would have a comparable effect when administered in a therapeutic dose. However, no significant effect of preoperative oral gabapentin on the onset or duration of the sensory and motor block during spinal anesthesia was observed.
CONCLUSION
Gabapentin administration (900 mg) in two divided doses within eight hours of surgery has no impact on the onset or duration of spinal anesthesia. It has a beneficial postoperative analgesic effect, reducing postoperative opioid consumption.
LIST OF ABBREVIATIONS
| VAS | = Visual Analog Score |
| OR | = Operating Room |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Institutional Review Board i.e. Ain Shams University Hospital (R 177/2022).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Written consent was collected from subjects or the next of kin.
STANDARD OF REPORTING
CONSORT methodology, as well as guidelines, were followed.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
FUNDING
None.
ACKNOWLEDEGMENTS
We would like to thank Mrs. Marwa Abolfotouh for her language editing and proofreading of the article. We would also acknowledge our fellow anesthesia residents who recruited the patients in our study and facilitated intra and postoperative data measurements.


