All published articles of this journal are available on ScienceDirect.
Breath-holding Test in the Prognosis of Postoperative Pain in Laparoscopic Gynecology: Observational Cohort Study
Abstract
Background
The problem of perioperative pain relief has not lost its relevance over the years. Studies have shown that patients report moderate to severe pain after surgery, even after laparoscopy. In recent years, specialists have focused on the role of baroreflex sensitivity in the functional state of the nociceptive and antinociceptive systems. Studies have shown that a test with maximum breath-holding during inspiration allows for a non-invasive and accurate assessment of the functional state of the cardiorespiratory system, making it possible to identify a cohort of patients with reduced baroreflex sensitivity
Objective
The aim of the study was to assess the relationship between the breath-holding test and postoperative pain and to develop a model for predicting pain after laparoscopic gynecological surgery.
Methods
Data from 489 patients undergoing gynecological laparoscopy at the Clinic of the Kuban State Medical University from August 2019 to September 2023 were analyzed.
Results
Severe postoperative pain was reported in 146 patients (29.9%). The duration of breath-holding was statistically significantly correlated with NRS upon admission to the PACU at all time points of the study (from -0,15 to -0,21). Logistic regression showed that the Generalized Anxiety Disorder scale score, Pain Catastrophizing Scale score, duration of surgery, Breath-holding duration, and endometriosis surgery influenced the risk of severe postoperative pain (NRS 7-10) (AUROC 0,809).
Conclusion
The breath-holding test, along with other factors, may be useful in assessing the risk of severe postoperative pain after laparoscopic gynecology.
1. INTRODUCTION
The problem of perioperative pain relief has not lost its relevance over the years. Studies have shown that between 30% and up to 80% of patients report moderate to severe pain in the days after surgery [1]. The evolution of minimally invasive methods in surgery, including for diagnosis and treatment, has led to a sharp and significant change in surgical practice in treating various types of diseases [2, 3]. Minimally invasive surgery, including laparoscopic surgery, has become widely accepted and has partly replaced traditional laparotomic surgical procedures in the treatment of various types of benign gynecological diseases [4].
Minimally invasive surgery, including gynecology, has a number of advantages, such as a significant reduction in surgical trauma, less severe pain associated with a postoperative wound, less use of systemic analgesics, better cosmetic results, shorter hospital stay, shorter recovery time and earlier return to daily activities and work compared to laparotomy [5, 6]. Despite these benefits, up to 80% of patients (35% to 80%) still experience severe pain after laparoscopic gynecologic surgery and require pain relief for mild to excruciating discomfort [7]. Pain syndrome after laparoscopic procedures has a specific pathophysiological mechanism, including inflammatory changes associated with surgical trauma and skin incision and morphological and biochemical changes in the peritoneum and diaphragm associated with pneumoperitoneum.
In recent years, great interest among specialists has been focused on the role of baroreflex sensitivity in the functional state of the nociceptive and antinociceptive systems. Increasing evidence suggests that baroreflex arc pathways also project to key central nervous system (CNS) that regulate somatosensory, somatomotor, and CNS arousal. In addition to maintaining autonomic homeostasis, baroreceptor activity modulates pain perception and neuroimmune, neuroendocrine, and cognitive responses to physical and psychological stressors [8]. The progression of chronic concomitant diseases is invariably accompanied by impaired baroreflex sensitivity (BRS) [9]. In recent years, leading researchers have shown increasing interest in determining the role of this factor in assessing the risk of unfavorable outcomes of the disease and the perioperative period [10-12]. Studies have shown that a test with maximum breath-holding during inspiration allows for a non-invasive and accurate assessment of the functional state of the cardiorespiratory system, making it possible to identify a cohort of patients with reduced baroreflex sensitivity [13].
The study aimed to assess the relationship between the breath-holding test and postoperative pain and to develop a model for predicting pain after laparoscopic gynecological surgery.
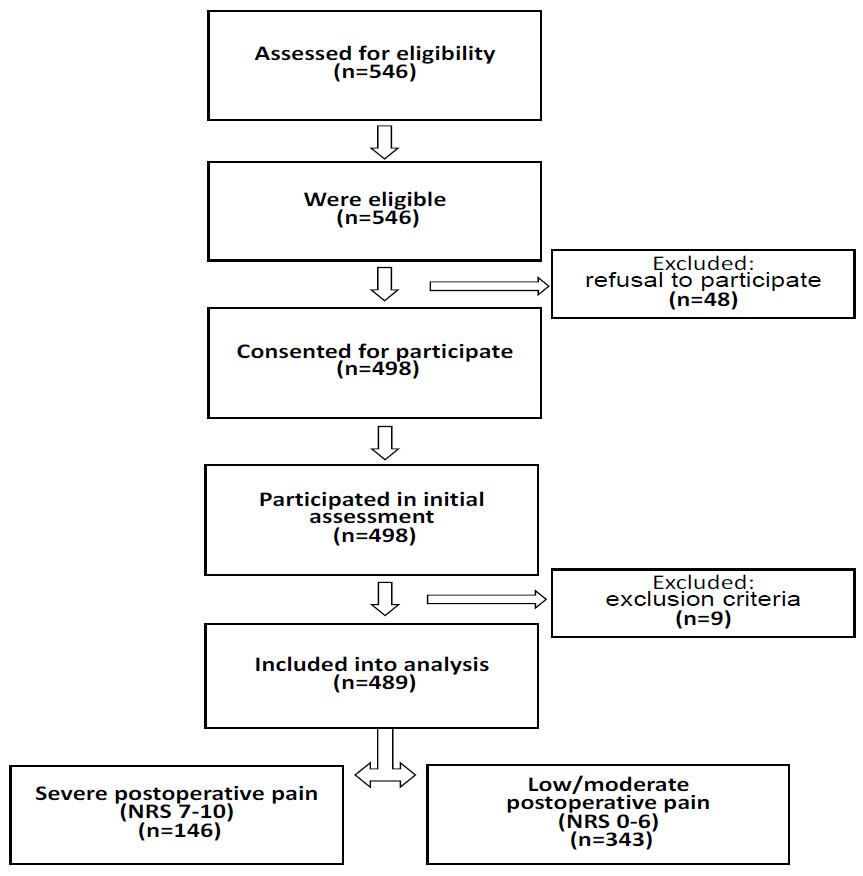
Note: NRS - numeric rating scale.
2. METHODOLOGY
We used a cohort study design to identify risk factors for the development of severe postoperative pain and the role of the breath-holding test in predicting pain after laparoscopic gynecology.
Data from 489 patients undergoing gynecological laparoscopy at the Clinic of the Kuban State Medical University from August 2019 to September 2023 were analyzed. Preoperatively, upon examination by the anesthesiologist, patients were sequentially assessed for inclusion in the study and, in the absence of exclusion criteria, were included in the study. Patients were followed up until postoperative day 2. This prospective clinical observational study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Local Ethics Committee in Kuban Medical University (No. 76 from 29.03.2019); patients signed informed voluntary consent before the recruitment to the study.
2.1. Inclusion Criteria and Patient’s Enrollment
All patients over the age of 18 years and undergoing laparoscopic gynecological surgery were assessed for inclusion in the study.
The exclusion criteria were incapacity to provide consent, emergency surgery, conversion to laparotomy, chronic substance use, history of psychiatric disease, pregnancy or lactation, severe chronic obstructive pulmonary disease, and body mass index of more than 30 kg/m2.
A total of 546 subjects were eligible, of whom 498 (91%) agreed to participate (Fig. 1). There were 9 (2%) drop-outs for various reasons: postoperative ventilatory support (n=3), complications followed by a second operation (n=4), and conversion to laparotomy (n=2). There were no missing data in outcomes and registered variables.
2.2. Preoperative Evaluation
The preoperative survey included questions about demographic information, including age, race, education, and marital status. It also asked questions about patients’ medical and surgical history, preoperative pain medication use, baseline pain, and expected pain after surgery (on a 0-10 numeric rating scale (NRS)). Lastly, all patients completed 3 validated instruments to measure depression (Patient Health Questionnaire (PHQ-9) [14]), anxiety (Generalized Anxiety Disorder 7-item scale (GAD7) [15]), and pain catastrophizing (Pain Catastrophizing Scale (PCS) [16]).
2.3. Breath-holding Test
The breath-holding test was performed as follows: voluntary breath-holding duration was assessed three times, with 10-minute intervals. After the deep inspiration of a volume equal to approximately 2/3 of the vital lung capacity, the participant was asked to hold their breath, and the duration of voluntary apnoea was measured from the beginning of the voluntary inspiration until reflex contractions of the diaphragm were noted by palpation. A mean value of the duration of the three samples was calculated.
2.4. Anesthesia and Pain Management
All patients underwent standard induction of anesthesia (propofol, fentanil, and rocuronium) and orotracheal intubation. Anesthesia was maintained with 4–6% desflurane (with the control of end-tidal anesthetic concentration) in air/oxygen (total flow, 0,5-1 L/min) to maintain a bispectral index between 40 and 60. Ventilation was controlled mechanically and adjusted to maintain an end-tidal carbon dioxide value between 30 and 40 mmHg throughout the surgery. Additional rocuronium and fentanyl were administered, as required. Laparoscopy was performed under video guidance, with three punctures in the abdomen. The trocar sites were pre-infiltrated with a 0.5% ropivacaine solution. The intraperitoneal pressure was maintained at approximately 12 mmHg. To prevent postoperative nausea and vomiting, all patients received 5 mg intravenous dexamethasone at the beginning of surgery and 75 μg intravenous ondansetron at the end of surgery. For postoperative pain control, multimodal analgesia, consisting of intravenous acetaminophen (500 mg) and ketorolac (30 mg), was administered 30 min before the end of surgery according to the national guidelines for postoperative pain management [17]. PCA was carried out by administering 0.1% morphine in boluses of 2 ml with a lockout of 5 minutes without any background infusion. PCA was started upon admission to the recovery room and stopped after the second-day visit, 24 hours after the end of surgery.
2.5. Study Outcomes and Time Points
Patients were assessed for NRS pain scores at the next time points: at the PACU (NRS-PACU), after 15 minutes (NRS 15 min), after 30 minutes (NRS 30 min), at a discharge from PACU (NRS ward), after 6 hours (NRS 6h) and at a study visit on the first postoperative day (NRS D2). Severe postoperative pain was stated if a pain corresponding to 7 or more points according to NRS at any time point was registered [18]. PCA therapy was ceased at postoperative day 2, and data on morphine consumption at 1 hour, 6 hours, and 24 hours postoperatively were extracted. To reduce the likelihood of biased assessment of postoperative pain, its determination was made by a specialist not involved in monitoring patients and prescribing medications.
2.6. Statistical Analysis
Based on planning for the inclusion of up to 5 risk factors in the final prognostic model and a severe postoperative pain rate of 30%, the required number of patients wasapproximately 500 patients. To assess the nature of the distribution, the Kolmogorov-Smirnov test was used. Data are presented as means, medians, and interquartile ranges. Categorical data were analyzed with Fisher’s exact test or χ2 test, and risk estimates were calculated using odds ratio and 95% confidence intervals (CIs). Continuous variables were assessed with the Mann–Whitney U test. Repeated measures analysis of variance (ANOVA) was used to determine differences in pain severity across time points. Spearman's correlation coefficient was used to assess the association of factors with pain severity. Logistic regression analysis was used to identify predictors of severe pain. The abilities of a model to predict severe acute pain (NRS 7–10) were measured using ROC analysis. Cut-off values used for the calculation of sensitivity and specificity were calculated based on goodness of fit (highest combined sensitivity and specificity).
| Parameter | All Patients (n=489) |
Severe Pain (n=146) | Low/moderate Pain (n=343) |
p |
|---|---|---|---|---|
| Age | 36 (30-45) |
35 (32-45) |
36 (31-47) |
0.454 |
| Weight (kg) | 63 (56-74) |
61 (52-73) |
64 (51-72) |
0.210 |
| BMI (kg/m2) | 26 (24-29) |
25 (22-28) |
26 (24-29) |
0.45 |
| ASA score | 1 (1-1) | 1 (1-1) | 1 (1-1) | 0.85 |
| Breath-holding test (sec) | 42 (36-48) | 39 (31-44) | 44 (38-49) | <0.0001 |
| PHQ-9 | 2 (2-3) | 2 (2-3) | 2 (1-3) | 0.0310 |
| GAD7 | 2 (2-3) | 2 (0-6) | 2 (0-3) | 0.0024 |
| PSC | 11 (7-12) | 11 (9-15) | 10 (7-12) | 0.0002 |
| Preoperative NRS | 1 (1-2) | 1 (1-2) | 1 (1-2) | 0.25 |
| Operation time | 50 (30-75) | 60 40-90) | 45 (30-65) | <0.0001 |
| Surgical Procedure | ||||
| Hysterectomy | 150 | 28 | 122 | 0,0003 |
| Endometriosis surgery | 112 | 72 | 40 | <0,0001 |
| Adnexectomy | 78 | 13 | 65 | 0,026 |
| Diagnostic laparoscopy | 65 | 11 | 54 | 0,016 |
| Myoma enucleation | 50 | 12 | 38 | 0,32 |
| Other | 34 | 10 | 24 | 0,95 |
| Time Point | Correlation Coefficient | CI95% | p |
|---|---|---|---|
| NRS-PACU | -0,18 | -0,26 - -0,10 | <0,0001 |
| NRS 15 min | -0,18 | -0,26 - -0,10 | <0,0001 |
| NRS 30 min | -0,18 | -0,26- -0,09 | <0,0001 |
| NRS ward | -0,15 | -0,223- -0,06 | 0,0007 |
| NRS 6h | -0,18 | -0,27 - -0,09 | <0,0001 |
| NRS D2 | -0,21 | -0,30 – 0,13 | <0,0001 |
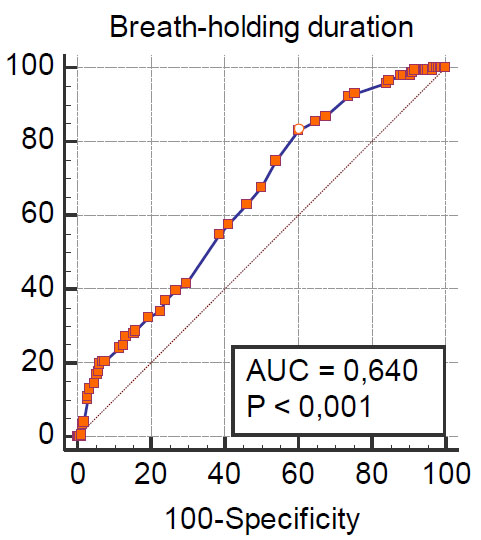
Note: AUC-area under the ROC curve.
3. RESULTS
A total of 489 patients were included in the analysis. Severe postoperative pain was reported in 146 patients (29.9%). Preoperative parameters for the study groups and the structure of surgical interventions are presented in Table 1.
The duration of the breath-holding test was statistically significantly lower in patients with severe postoperative pain. Also, its duration was statistically significantly correlated with NRS upon admission to the PACU at all time points of the study (Table 2).
However, ROC analysis showed that the breath-holding test alone has insufficient predictive value (Fig. 2).
The logistic regression performed allowed us to identify factors influencing the risk of developing severe postoperative pain. These include the duration of the breath-holding test, scores on the PSC and GAD7 scales, the duration of the surgery, and the type of operation - endometriosis surgery. Table 3 shows the logistic regression equation (Nagelkerke R2 0.37).
The Hosmer–Lemeshow test showed a goodness of fit for the logistic regression model (Chi-squared 5.4, P = 0.7110). ROC analysis showed a good prognostic value for the model (area under the ROC curve (AUC) 0.809, standard error 0.022, 95% confidence interval 0.771 to 0.842). ROC are shown in Fig. (3).
The cut-off point was >39,1% for the risk of severe postoperative pain (sensitivity 62,33% and specificity 87,76%).
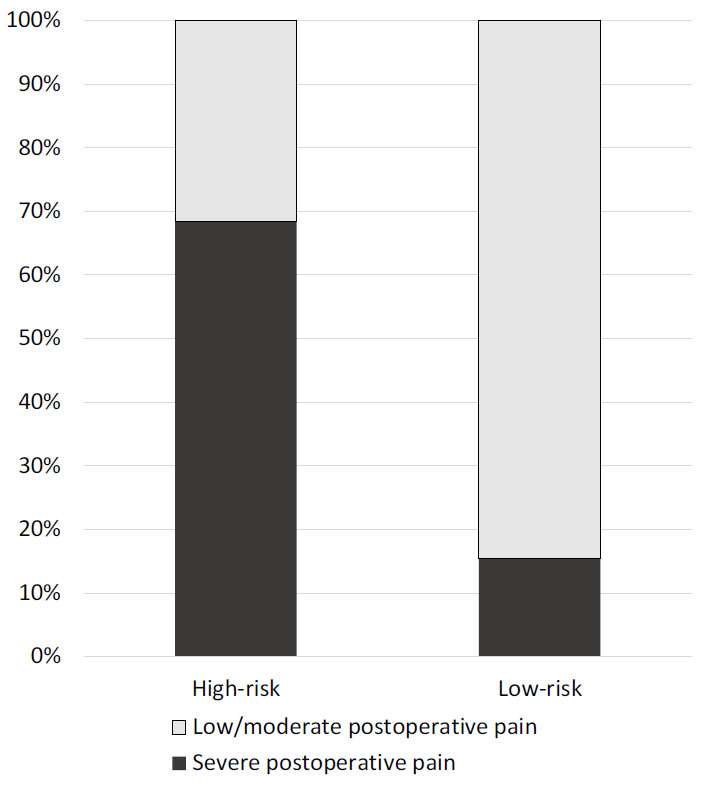
Retrospectively, all patients in the study cohort were divided into two groups according to the developed model - patients with a high (>39.1%) and low risk (39.1% or less) of severe postoperative pain. The proportion of patients with NRS 7 or more was 15.4% in the low-risk group and 68.4% (<0.0001) in the high-risk group (Fig. 4). High-risk and low-risk patients were different in GAD7 (2(2-4) vs. 2 (2-2), p<0.0001), PSC (12 (10-17) vs. 11 (8-12), p<0.0001), breath-holding duration (44 (43-47) sec vs. 38 (35-40) sec, p<0.0001), duration of procedure (45 (30-55) min vs. 65 (50-75) min, p<0.0001) and endometriosis rate (18% vs. 35%, p<0.0001). PHQ-9 (2 (2-2) vs. 2(2-2), p=0.26), preoperative pain ((1 (1-2) vs. 1 (1-2) and age (35 (31-44) vs. 36 (32-46), p=0.47) were comparable between high-risk and low-risk groups.
| Variable | Coefficient | Std. Error | P | Odds ratio | 95% CI |
|---|---|---|---|---|---|
| PSC score | 0,087674 | 0,023757 | 0,0002 | 1,0916 | 1,0420 -1,1437 |
| GAD7 score | 0,10992 | 0,030379 | 0,0003 | 1,1162 | 1,0517 - 1,1847 |
| Duration of surgery, min | 0,010172 | 0,0033701 | 0,0025 | 1,0102 | 1,0036 - 1,0169 |
| Breath-holding test, sec | -0,050749 | 0,011806 | <0,0001 | 0,9505 | 0,9288 - 0,9728 |
| Endometriosis surgery | 1,88334 | 0,26565 | <0,0001 | 6,5754 | 3,9066 - 11,0675 |
| Constant | -1,25155 | 0,59075 | 0,0341 | - | - |
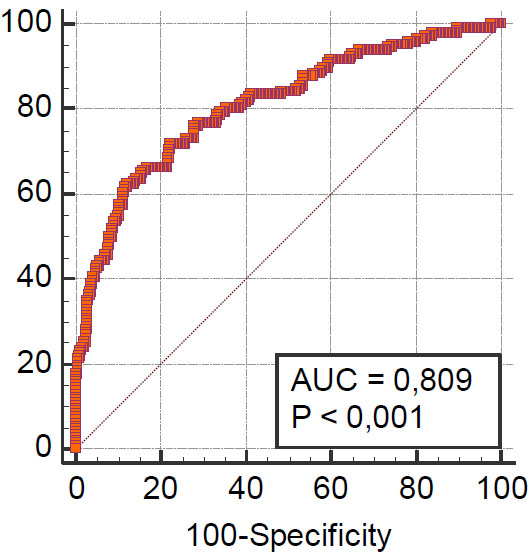
Note: AUC-area under the ROC curve.
| - | All Patients | Low-risk Group | High-risk Group | - | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time point | Mean | Median | 95% CI for median | Mean | Median | 95% CI for median | Mean | Median | 95% CI for median | Р between two groups |
| NRS-PACU | 4,39 | 4 | 3-4 | 3,75 | 3 | 3-3 | 6,12 | 6 | 6-7 | <0,0001 |
| NRS 15 min | 4,79 | 4 | 4-4 | 4,14 | 4 | 4-4 | 6,55 | 7 | 6-7 | <0,0001 |
| NRS 30 min | 4,51 | 4 | 4-4 | 3,99 | 4 | 3-4 | 5,90 | 6 | 5-7 | <0,0001 |
| NRS ward | 3,72 | 3 | 3-4 | 3,29 | 3 | 3-3 | 4,87 | 5 | 5-5 | <0,0001 |
| NRS 6h | 2,98 | 3 | 2-3 | 2,62 | 2 | 2-2 | 3,94 | 4 | 4-4 | <0,0001 |
| NRS D2 | 2,43 | 2 | 2-2 | 2,16 | 2 | 2-2 | 3,18 | 3 | 3-3 | <0,0001 |
Pain levels also varied between groups. At all time points, the NRS score was statistically significantly higher in the high-risk group (Table 4).
Median morphine consumption at 1 h postoperatively was 5,5 mg (3,9–8,7) in the low-risk group and 7.4 mg (5.1–10.1) in the high-risk group (p = 0.006) and was also significantly different at 6 h (11.2 (8.3–18.0) vs. 17.0 (10.2–24.7) mg; p = 0.001) and on postoperative day 2 (19.0 (11.0–30.1) vs. 26.0 (17.0–38.2) mg; p = 0.003), respectively.
4. DISCUSSION
Our data indicate that the incidence of severe postoperative pain is 29.9% in laparoscopic gynecology, while the duration of the breath-holding test has a certain prognostic value in assessing the risk of its development and, along with other predictors, can be useful in clinical practice.
Although minimally invasive surgery has traditionally been presented as an area with less postoperative pain, this is not always true. As shown by a study by Gerbershagen et al. [18], laparoscopic gynecological surgery was associated with a relatively high level of pain (median NRS 5 (3-7)) and a high need for opioids, practically not inferior to the pain after open operations and exceeding the pain from, for example, open right hemicolectomy. Also, laparoscopic gynecological surgeries have been associated with more pain than laparoscopic surgical procedures (e.g., cholecystectomy). In addition, pain after laparoscopic surgery is often underestimated, and in this category of patients, opioids are used much less frequently for the treatment of postoperative pain. Previous studies have shown that risk factors include female gender, young age, and preoperative pain [20-22], which partly explains the authors' findings. However, even within this population, the pattern of postoperative pain severity is varied, and there are other factors that influence its intensity. Our study showed that pain is relatively high in laparoscopic gynecology, even despite the use of opioids. The median NRS in the overall group was 4 (2-6) on admission to the PACU, which is slightly less than in the study by Gerbershagen HJ et al. [18] and is explained by the variety of procedures in our research. High-risk patients had higher pain intensity at comparable ages and preoperative pain levels.
The type of surgical intervention, as indicated above, was often identified as a predictor of severe postoperative pain. In our study, the type of laparoscopic gynecology was included in the regression model, and only endometriosis surgery had a significant effect on postoperative pain. This fact can hardly be considered surprising; endometriosis is a complex disease that is often associated with pain, including chronic pain. This is a common gynecological disease with an incidence of up to 10% in fertile women. Its development is associated with the presence of endometrium outside the uterine cavity; the disease has its own characteristic neuroendocrine and inflammatory features [23, 24]. The pattern following from our results is different from the data obtained by Kanellos et al. [25], who concluded that it is preoperative pain, and not endometriosis itself, that increases the severity of postoperative pain. Van Aken et al. [26], however, believe that the pathogenesis of pain in endometriosis has its own specifics, and women suffering from this disease are less tolerant of painful sensations in the pelvic area, which may be the reason for more severe pain in the postoperative period. Length of surgery has also been identified as a predictor of severe postoperative pain. An increase in pain over time may be due to greater trauma, as well as the influence of infiltration anesthesia, the effect of which is limited in time. Suragul et al. showed that the effect of local anesthesia at trocar sites is significantly reduced if the duration of anesthesia exceeds 2 hours [27].
The role of psycho-emotional factors in the severity of postoperative pain plays a significant role in gynecology. There were significant correlations between baseline pain and psychological measures of distress pre-operatively [28]. Wong et al. also found that the PHQ-9, GAD-7, and PCS scales are associated with the severity of postoperative pain [29]. Catastrophizing pain is often a factor not only in severe postoperative pain but also a predictor of the transition from acute pain to chronic pain in various areas of surgery, including gynecology [30-32]. In our study, only GAD-7 and PCS were associated with pain. These data suggest that the psychoemotional component of pain is an important factor and a potential target for multimodal analgesia methods used in the perioperative period.
To our knowledge from the literature review, this is the first study to evaluate the ability of the breath-holding test to determine the risk of postoperative pain, and it showed that the duration of the breath-holding test is negatively correlated with pain severity.
The role of baroreflex blood pressure control in perioperative outcomes has recently received much attention. Baroreflex dysfunction before surgery is detected quite often, especially in patients with a number of concomitant cardiac and respiratory diseases, such as hypertension [33], diabetes [34], carotid atherosclerosis [35, 36], obesity [37], in smokers [38], high alcohol consumption [39] and obstructive sleep apnea [40]. Baroreflex dysfunction and autonomic nervous system imbalance modulate local and systemic inflammatory processes in animal experiments [41]. Autonomic regulation of inflammatory mediators acts directly on nociceptors or indirectly on sympathetic nerve endings, producing and releasing inflammatory substances that contribute to pain perception [42]. In addition, afferent pain signaling may directly modulate other components of inflammation, including plasma extravasation and neutrophil function, which are modulated by vagal afferent activity [43]. The role of the sympathetic nervous system in the development of hyperalgesia has also been demonstrated in humans [44]. Several clinical studies have demonstrated the role of baroreflex sensitivity on pain severity. Thus, a prospective study found that low baroreflex sensitivity was associated with a higher NRS score in the first 6 weeks after hand surgery [45]. There is also work indicating that preoperative resting blood pressure and, presumably, arterial baroreflex sensitivity is associated with the intensity of postoperative pain at 24 hours and 48 hours after prostatectomy and after cesarean section [46, 47]. The relationship between the baroreflex and pain is discussed in more detail in the review by Suarez-Roca H et al. [8]. As fundamental research has shown, the breath-holding test reflects the sensitivity of the baroreflex [13], which explains the patterns obtained in our study.
Limitations. The breath-holding test is a surrogate marker of the functional state of the reflex regulation of the cardiorespiratory system and may also reflect the psycho-emotional state of patients. However, in the present work, it was shown to be a factor independently influencing the level of pain. Secondly, the study was conducted at a single center, and anesthesia was provided by different specialists whose contribution was not assessed, and these results need external validation. The obtained patterns reflect the characteristics of postoperative pain syndrome in laparoscopic gynecology. For open operations, the analgesia strategy may be different, as well as the duration of the operation. On the other hand, laparoscopic operations in other areas of surgery may also have characteristics associated with the nature of the underlying disease, gender, and age characteristics.
CONCLUSION
The breath-holding test, along with other factors, may be useful in assessing the risk of severe postoperative pain after laparoscopic gynecology. Factors such as the duration of the operation, endometriosis surgery, the degree of inclusion of emotional and behavioral components in the structure of the pain syndrome according to the pain catastrophizing scale and anxiety, which we determined using the GAD-7 scale, also contribute to the development of severe pain.
LIST OF ABBREVIATIONS
| AUC | = Area under the curve |
| PACU | = Post-anesthesia care unit |
| ROC | = Receiver Operator Characteristic |
| GAD-7 | = Generalized Anxiety Disorder 7-item scale |
| NRS | = Numerical Rating Scale |
| PCS | = Pain Catastrophizing Scale |
| PHQ-9 | = Patient Health Questionnaire |
| CNS | = Central Nervous System |
| BRS | = Baroreflex Sensitivity |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This prospective observational study was conducted after receiving approval from the local ethics Committee at Kuban Medical University (No. 76 from 29.03.2019).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.


