All published articles of this journal are available on ScienceDirect.
Comparison between Pressure Recording Analytical Method and Fick Method to Measure Cardiac Output in Pediatric Cardiac Surgery
Abstract
Background:
There is an increased interest in methods of objective cardiac output measurement in pediatric cardiac surgery. Several techniques are available, but have limitations, among the new technologies pressure recording analytical method with MostCare (MostCare-PRAM), a minimally invasive hemodynamic monitoring system, represents a novel arterial pulse contour method that does not require calibration. For this reason, we compared the MostCare-PRAM vs the Fick method for estimation of cardiac output.
Methods:
We studied prospectively 13 pediatric patients who underwent cardiac surgery and compared intraoperatively Cardiac Index (CI) measured with the MostCare-PRAM with the CI measured with the Fick method. We also measured Cardiac Cycle Efficiency (CCE) and maximal arterial pressure/time ratio (dp/dt max) and compared with Fick method.
Results:
The data showed good agreement between CI Fick and CI MostCare-PRAM (r = 0.93 and R2= 0.86; p < 0.0001) and also between CCE (r = 0.82 and R2 = 0.67; p < 0.001) and dp/dt (r = 0.84; R2 = 0.81; p < 0.001) with CI measured with Fick method.
Conclusion:
In pediatric patients submitted to cardiac surgery, the MostCare-PRAM seems to estimate CI with a good level of agreement with the Fick method measurements.
1. INTRODUCTION
It is well known that clinical assessment of cardiac output using indirect parameters of systemic blood flow is beneficial for the patient and reduces morbidity and mortality. In children submitted to cardiac surgery, low cardiac output is associated with an increased mortality [1] and any delay in the diagnostic process of shock increases mortality [2] to prevent the risks of low systemic blood flow by monitoring cardiac output. Many methods of cardiac output monitoring are available, but not all are feasible in the pediatric population. This limitation is due to technical and size restraints, the potential toxicity of indicators (lithium, carbon dioxide), risk of fluid overload, difficulties in vascular access and, above all, the presence of shunts (transitional circulation, congenital heart defects) [3].
Between the various methods PRAM (Pressure Recording Analysis Method) is a method designed for arterial pressure-derived continuous CO and it is the only methodology that does not need any starting calibration, central venous catheterization, or any adjustments based on experimental data but only an arterial line (radial, brachial, femoral) [4]. The aim of this study was to evaluate the reliability of an uncalibrated pulse contour method, PRAM, to measure CO in pediatric patients scheduled for cardiac surgery, compared with the Fick method. Our hypothesis is that PRAM will show a good correlation with the Fick method for determining cardiac index in pediatric patients.
2. MATERIAL AND METHODS
After obtaining approval of the local Ethics Committee and parental informed consent, we studied prospectively 13 children with congenital heart disease with a mean age of 1 year and 8 months and weight of 11.5 kg (± 12.6 SD), undergoing elective congenital heart surgery. All the patients were performed with Cardiopulmonary Bypass (CPB). According to the standard institutional practice, after premedication with 0.5 mg/kg oral midazolam, anesthesia was induced with midazolam 0.1 mg/Kg, fentanyl (3 μg/kg) and subsequently maintained and balanced with fentanyl (3-6 μg/kg/h) and sevoflurane (1-1.5 vol %) inhalation. During CPB sevoflurane was substituted with propofol at 3-5 mg/Kg/h. Muscle relaxation for intubation was achieved with 0.1 mg/kg rocuronium and maintained with a dosage of 0.05 mg/kg/h. After intubation, the patients were ventilated with a fixed tidal volume of 10 ml kg and a frequency adapted to age and to maintain the PaCO2 between 30 - 40 mmHg. The Positive end-expiratory pressure was set between 3 and 5 cm H2O and FIO2 maintained between 30 and 50%. A 4–5.5 Fr central venous catheter was inserted in the right internal jugular vein or alternatively in the right or left subclavian vein. An arterial line was inserted percutaneously using a 22 or 24 gauge cannula. We evaluated with Transesophageal Echocardiography (TEE) all the patients using a pediatric VO2
Exclusion criteria were: atrial fibrillation and/or ventricular arrhythmias, the acute need for inotropic drugs after induction of anaesthesia, pathologies that could affect the quality and reliability of the arterial signal due to dicrotic notch disturbances, poor quality of the arterial pressure signal after a standard flush test and lung disease and pleural effusion. During Cardiopulmonary Bypass (CPB), deep or moderate hypothermia was reached according to the type of surgery. At the end of CPB most patients received inotropic support consisting of milrinone (0.75 μg/kg/min), dopamine (3–6 μg/kg/min) and epinephrine (0.05–0.15 μg/kg/min) or norepinephrine (0.05-0.15 μg/kg /min) when necessary. In patients with increased pulmonary vascular resistance, Nitric Oxide (NO) was administered at the dose from 20 to 40 PPM. To measure cardiac output, we used MostCare (powered by PRAM) that is a minimally invasive hemodynamic monitoring system based on mathematical analysis of the radial, brachial or femoral arterial waveform recorded at a high sampling rate (1000 Hz) [5]. PRAM permits by mathematical analysis of the changes in the contour of the arterial pressure waveform to assess beat-by-beat the stroke volume from the arterial pressure waveform. The arterial waveform is analysed after the monitor is connected to an arterial line via a standard pressure transducer. With the PRAM, is possible to measure not only Cardiac Output (CO) and Cardiac Index (CI), but also Cardiac Cycle Efficiency (CCE), Arterial Elastance (Ea), maximal arterial pressure/time ratio (dp/dt max), Systemic Vascular Resistance Index (SVRI), Stroke Volume Variation (SVV), Pulse Pressure Variation (PPV), where CCE represents the ratio between hemodynamic work performed and energetic expenditure for the maintenance of homeostasis, Ea the arterial elastance that is the ratio between end-systolic pressure and stroke volume, the dP/dt as the maximal rate of pressure changes over time measured between 2 consecutive points during systolic upstroke,
The measurements were recorded every 15 seconds for the time from the beginning of operation to the end of the surgery, avoiding the CPB period.
We compared this system with the Fick method, that is regarded as the standard of reference in cardiac output monitoring in a research setting, despite its limitations due to the need of sampling of mixed venous blood [3].
By the Fick method, the cardiac output is calculated by measuring the oxygen content of venous blood and arterial blood (in ml/l) and hence measuring the difference between the two, which represents the tissue oxygen utilisation. In our case, as is common, we estimated oxygen consumption (VO2) (in a pediatric patient usually is 160 ml/min/m2) then we calculated cardiac output by the simple equation: CO = VO2/(arteriovenous O2 difference) where CO = blood flow in l/min:
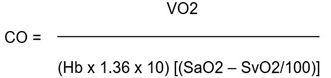 |
and Cardiac Index (CI):
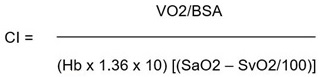 |
where SaO2 = arterial saturation, SvO2 = mixed venous saturation; BSA = Basal Surface Area.
However, calculation of cardiac output by the Fick method represents a valid method for use in patients with septal defects and associated shunts. Infact, the same equation allows calculation of either pulmonary blood flow or systemic cardiac output-by substituting O2 difference (SaO2-SvO2) as:
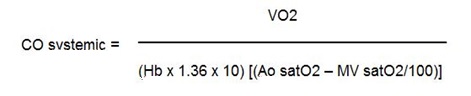 |
 |
where MV = Mixed Venous Saturation; PV = Pulmonary Vein Saturation; PA = Pulmonary Artery Saturation.
Similarly, effective flow may be estimated, employing the difference in oxygen content between the pulmonary vein and a “mixed venous” sample (systemic AV O2 difference).
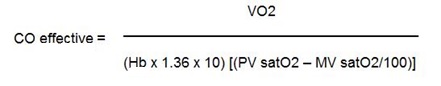 |
To measure the effective CO, the mixed venous saturation was measured using mixed venous blood sampled by the surgeon after heparinization of the patients before CPB and pulmonary veins saturation in the same way by the surgeon. We did not use left atrial blood samples to measure pulmonary veins saturation because it could be affected in presence of intracardiac shunts and the patients did not have pulmonary problems.
For mixed venous saturation, the tradition is to use the most distal right heart chamber or site where there is no left to right shunt. Thus, right atrium may be used in the absence of an atrial septal defect or right ventricle if there is no shunt at atrial or ventricular level. In Practice Superior Vena Cava (SVC) saturation is often used but a value intermediate between SVC and Inferior Vena Cava (IVC) may be preferable as the two may be significantly different. It has been demonstrated that the mixed venous saturation more closely approximates to the SVC than to the IVC. Hence the following formula: MV Sat = (3 Sat SVC + 1 Sat IVC)/4 we used to measure mixed venous saturation with blood sampled by the surgeon [6].
Because MostCare-PRAM is able to estimate the beat-to-beat CO, both measurements, PRAM-CI and Fick-CI, were acquired simultaneously, and exactly PRAM-CI values as the mean value when SVC, IVC and pulmonary vein blood samples were collected.
The results are expressed as means ± Standard Deviations (SD). The relationships between parameters were assessed by Pearson correlation (r) and calculating the linear regression analysis with the Pearson’s coefficient expressed as the R2 value with 95% confidence interval (95% CI). Bland–Altman analysis, a method of data plotting used in analyzing the agreement between two different assays, was performed to compare effective CI values obtained by Fick vs PRAM and other parameters, calculating the bias as the mean difference between measurements.
The limits of agreement were calculated as the mean ± 1.96 SD from the bias, defining the range in which 95% of the values are expected to fall. A value of p < 0.05 was considered statistically significant. For statistical evaluation, MedCalc 12.7.0.0 for Windows was used.
3. RESULTS
Thirteen among neonates, infants and pediatric patients were enrolled in this study. Details of the patient characteristics, including sex, age, weight, height, BSA and Congenital Heart Disease (CHD) are reported in Table 1. The mean age of the patients was 1 year and 8 months, and the weight ranged between 3.1 to 53 Kg (mean 11.5 kg ± 12.6). Between all the patients, 1 had bi-directional shunt, 3 with right to left shunt, 6 with left to right shunt and 3 without intracardiac shunt. Each patient was successfully transferred in ICU at the end of the surgery and no complications have been found during our follow-up. During the study period the hemodynamic value of all patients are reported in Table 2 with mean value ± SD. In all patients, it was possible to measure effective cardiac output with Fick method and, as reported before, we avoided patients poor quality of the arterial pressure signal after a standard flush test.
| Patient n° | Female/Male | Age (days – months-years) | Weight (Kg) | Height (cm) | BSA (m2) | Diagnosis of CHD |
|---|---|---|---|---|---|---|
| 1 | M | 4 y | 18 | 107 | 0.73 | PS |
| 2 | F | 9 d | 3.1 | 50 | 0.18 | TGA |
| 3 | F | 1 y 6m | 11.7 | 88 | 0.52 | AS |
| 4 | M | 1 y 6 m | 9.0 | 83 | 0.45 | MS |
| 5 | M | 3 m 20 d | 4.2 | 58 | 0.25 | CAVC |
| 6 | M | 4 m 10 d | 6.2 | 65 | 0.32 | TOF |
| 7 | F | 8 m 5 d | 6.9 | 68 | 0.35 | VSD |
| 8 | F | 3 y | 11.7 | 90 | 0.53 | CAVP |
| 9 | M | 12 y | 53 | 173 | 1.63 | ASD |
| 10 | F | 5 m 6 d | 5.0 | 61 | 0.28 | VSD |
| 11 | F | 7 m 21 d | 8.7 | 65 | 0.37 | TOF |
| 12 | M | 4 m 5 d | 4.3 | 61 | 0.26 | VSD |
| 13 | M | 9 m 15 d | 7.4 | 67 | 0.35 | TOF |
| Patient | HR | Ea | SVRI | PPV | SVV | CCE | dp/dt max | CI Mostcare | CI Fick |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 93 | 8,5 | 1981 | 24 | 13 | -1,1 | 0,49 | 1,5 | 1,4 |
| 2 | 110 | 5,4 | 1507 | 47 | 13 | -0,84 | 0,58 | 1,8 | 1,6 |
| 3 | 102 | 5,7 | 1359 | 33 | 51 | -0,89 | 0,78 | 2,2 | 2,2 |
| 4 | 138 | 7,1 | 1331 | 33 | 19 | 0,2 | 1,32 | 3,3 | 3,6 |
| 5 | 139 | 8,3 | 1483 | 39 | 20 | 0,16 | 1,27 | 3,3 | 3,8 |
| 6 | 138 | 8,5 | 1418 | 48 | 19 | -0,17 | 0,74 | 2,7 | 2,2 |
| 7 | 140 | 8,8 | 1426 | 36 | 15 | -0,17 | 0,88 | 3,4 | 3,2 |
| 8 | 104 | 8,1 | 1353 | 40 | 45 | -0,25 | 0,57 | 2,6 | 2,5 |
| 9 | 91 | 6,2 | 1653 | 40 | 33 | -0,73 | 1,02 | 2,4 | 2,5 |
| 10 | 128 | 6,8 | 1565 | 50 | 39 | -0,29 | 0,93 | 2,0 | 2,2 |
| 11 | 145 | 8,1 | 1480 | 37 | 45 | -0,25 | 0,57 | 2,1 | 2,1 |
| 12 | 152 | 9,4 | 1495 | 65 | 41 | -0,2 | 0,75 | 2,4 | 2,8 |
| 13 | 149 | 7,5 | 1347 | 43 | 35 | 0,05 | 0,99 | 2,9 | 2,9 |
| Mean±SD | 125±22 | 7,6±1,2 | 1492±174 | 41±10 | 30±14 | -0,34±0,41 | 0,84±0,26 | 2,5±0,6 | 2,7±0,7 |
Systolic blood pressure values ranged from 54 to 98 mmHg with a mean value of 76 mmHg. HR from 91 to 144 beats per minute, with a mean value of 124. Effective CI by Fick method with values from 1.4 to 3.8 l/min/m2, and CI by MostCare-PRAM with values from 1.5 to 3.3 l/min/m2, the mean value did not differ between the two methods (respectively 2.53 to 2.51 l/min/m2). The correlation analysis with the Pearson’s coefficient (r= 0.93 and R2= 0.86; p < 0.0001) is shown in Table 3 and Fig. (1a). The Bland–Altman methods Fig. (2a) exhibited a good agreement between CI Fick and CI MostCare-PRAM.

| R | R2 | p-value | |
|---|---|---|---|
| CI Mostcare | 0.93 | 0.86 | < 0.0001 |
| dP/dt max | 0.84 | 0.71 | < 0.001 |
| CCE | 0.82 | 0.67 | < 0.001 |
| SVV | -0.07 | 0.005 | NS |
| PPV | 0.06 | 0.004 | NS |
| SVRI | -0.54 | 0.28 | NS |
| Ea | 0.30 | 0.09 | NS |
| HR | 0.55 | 0.3 | NS |
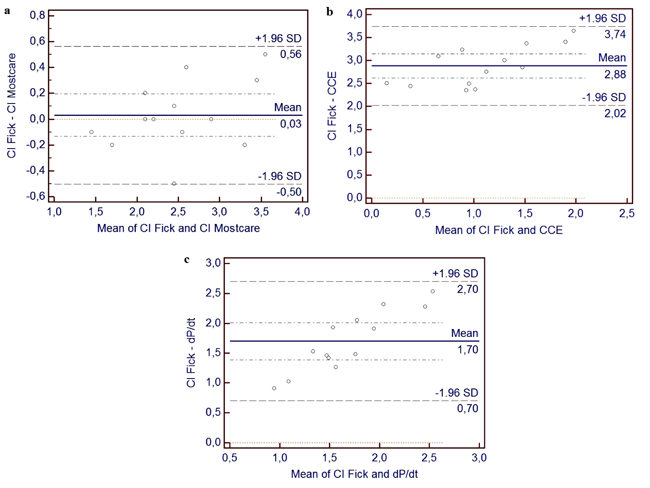
We also observed the correlation with other parameters, where we have found a correlation and regression between CI by Fick with CCE (r = 0.82; R 2 = 0.67; p < 0.001) (Table 3), (Figs. 1 and 2). In addition, we found a significant correlation and regression (r = 0.84; R2 = 0.81; p < 0.001) between CI by Fick with dp/dt. No correlation was found between CI by Fick method with other parameters measured with Mostcare-PRAM.
4. DISCUSSION
The measurement of Cardiac Output (CO) is of considerable interest in ill-pediatric patients and in many states of disease, including surgery for congenital heart disease [7-9]. Maintenance of adequate organ substrate delivery is important in these kind of patients to reverse or prevent ischemic injury, which can result in multi-system organ dysfunction, prolonged morbidity, and mortality [10-12]. Because interventions targeting early treatment of low cardiac output have improved outcome in surgical patients [13], detection of inadequate oxygen delivery through hemodynamic assessment is important for preventative or therapeutic interventions and adequate organ perfusion is the goal of supportive and therapeutic intensive care.
Blood pressure and other standard monitors provide inadequate indication of low cardiac output, especially in children, and are worse indicators of the distribution of cardiac output. Monitors that more directly assess organ oxygenation offer the possibility of improved recognition and treatment of circulatory abnormalities to reduce multiorgan dysfunction and related morbidity and mortality [14].
Between the technologies, thermodilution using the pulmonary artery catheter or transpulmonary thermodilution has been most commonly used to measure cardiac output.
In pediatric surgical patients, these techniques are not routinely used due to their increased risk of complications (infections, thrombosis, embolism), size of the catheter which is difficult to insert in smaller patients, so that it cannot be used with small children, infants, and babies [15], and those with aberrant cardiopulmonary anatomy [16] due to the presence of intracardiac shunts where is not recommended if intra- or extracardiac shunts are present [17, 18].
Between other less invasive methods that have been developed to measure CO, other indicator dilution techniques that use several indicators, such as indocyanine green or lithium, although are less invasive in avoiding for example the need for pulmonary artery catheterization, is restricted by the accumulation of indicator in some cases and mostly by the presence of intracardiac shunts.
Among the other approved methods, arterial pressure contour analysis (pulse contour method) permits to measure Stroke Volume (SV), required to calculate CO, on a beat to-beat basis from the arterial pulse pressure waveform. With an arterial pressure waveform of sufficient fidelity, stroke volume can be measured from an algorithm that uses pulse area and morphology [19]. There is no linear relationship between pressure and flow in the aorta, which is primarily due to aortic root impedance, aortic compliance and systemic vascular resistance. This implies that pulse contour analysis can be used to detect changes in cardiac output and that the measurements generally need to be calibrated with another technology, such as transpulmonary thermo- or lithium dilution. Despite this method is widely used in adults, its use in pediatric patients has scant reliability due to the use of nomograms that are adapted for adults, and due to the impossibility or limited repeatability of calibration for thermodilution techniques in small children.
In this work, the method that we used was described in 2002 by Romano et al. [4]. It consists of a method for measuring CO based on the analysis of the arterial pressure waveform with an algorithm that could be used in pediatrics. This new method called PRAM is based on the mathematical analysis of the changes in the contour of the arterial pressure waveform. In particular, MostCare, powered by the Pressure Recording Analytical Method (PRAM), is a hemodynamic monitoring system based on the analysis of arterial waveform (including the postdicrotic notch phase) recorded at a high sampling rate (1000 pressure/time points) [4].
It facilitates the beat-by-beat assessment of the SV from the arterial pressure waveform from a radial, brachial, or femoral artery and does not require external calibration or preloaded anthropometric patient data. The arterial waveform is analysed and beat-by-beat data are delivered after the monitor is connected to an arterial line via a standard pressure transducer. This method has been widely studied both in animals and in adult patients [20-23]; nevertheless, the limited number of published studies are on pediatric patients [24-28]. Although some published results from using PRAM in the paediatric setting have been controversial [28-31], there is a strong rationale for using an uncalibrated pulse contour method such as PRAM in paediatric cardiac surgery, because it can be used regardless of the patient’s age (no need for dedicated arterial cannulas) and the underlying anatomy (e.g. the presence of intracardiac shunts or a univentricular heart). In our case, we conducted a prospective analysis of haemodynamic monitoring in neonates and infants admitted to cardiac surgery. The aim was to verify whether the CI estimated by PRAM was associated with a good agreement with CI measured by the Fick method in paediatric patients undergoing cardiac surgery on CPB for congenital heart disease also with intracardiac shunts and whether it could therefore be considered a clinically reliable monitoring method. This is the first validation study in this setting to compare MostCare-PRAM with a reference method and like Critchley and Critchley [32], we used the Bland– Altman method [33] for additional CO comparison between the two methods.
The results of our study showed that CI measured with MostCare–PRAM has a good level of agreement as compared by Fick method. Actually only few studies have been carried out on MostCare-PRAM in pediatric patients with favourable results [24-28]. Alonso [24] demonstrated that in pediatric patients undergoing diagnostic right and left heart catheterization, the MostCare-PRAM was shown to estimate CI with a good level of agreement with the Fick method measurements. In Garisco [25] PRAM was compared to bioreactance that provided similar CI estimation at stable hemodynamic conditions in pediatric cardiac surgery. In particular, he evaluated the difference between Stroke Volume Index (SVI) measured by PRAM and bioreactance and their ability to track changes after the treatment of children undergoing cardiac surgery. They observed that under stable hemodynamic conditions, both methods provided similar SVI estimations, although bioreactance SVI values appeared significantly lower than PRAM after fluid replacement. Taking into account that it is not a validation study, it underlines the importance of the evaluation of hemodynamic parameters as guidelines for fluid therapy in pediatric patients. Another study compared CO between Mostcare-PRAM and Doppler echocardiography in critically ill non-cardiac patients [26]. The results showed a good agreement between both methods.
Finally, in a further study, CI estimated by PRAM after paediatric cardiac surgery was reliably associated with clinical indicators of tissue perfusion, with vasoactive and diuretic drug requirements, and predicted longer mechanical ventilation duration [28].
The results of our study confirm the good level of agreement and interchangeability of CI values obtained with the MostCare-PRAM method and the CI values obtained using Fick principle as a standard of reference, regardless of age and weight and the underlying anatomy (e.g. the presence of intracardiac shunts or a univentricular heart). This is the first one in this sense, where we also used the Bland–Altman method for additional CI comparison between the two methods. In fact Bland and Altman make the point that any two methods that are designed to measure the same parameter (CI) should have a good correlation when a set of samples are chosen such as the property to be determined varies considerably. One primary application of the Bland–Altman plot is to compare two clinical measurements that each provides some errors in their measurements, and also can be used to compare a new measurement technique or method with a standard of reference, even a standard of reference does not - and should not - imply it to be without error.
About CCE and dp/dt, the correlation with CI measured by Fick method demonstrated that both parameters are related to the cardiac function so that an increase in efficiency or contractility is correlated with an increase in cardiac output. No other analysis was performed with respect to the other hemodynamic parameters. The aim of our study was to evaluate the reliability of the method in the patient's hemodynamic study. However, a possible limitation of the method should not be forgotten particularly in pediatric patients that is important to mention. In some clinical situations, especially when vasoconstriction is present, the dicrotic notch is not clearly observed in the arterial pressure curve. In these circumstances, the PRAM method is able to detect the point of instability that corresponds to the dicrotic notch by sampling the arterial signal at 1000 Hz, and thereby estimate the SV [4, 34]. Another clinical issue that must be addressed and represent an advantage of MostCare-PRAM is the possibility to avoid complications due to arterial catheterization. Although this is a problem related to the technique and not directly to the method, the ability to connect the MostCare-PRAM to any arterial catheter can be an advantage over transpulmonary thermodilution that requires a femoral artery cannulation and a specific catheter.
Our study has limitations. The study population comprised a limited number of pediatric patients, and the congenital cardiac abnormalities were heterogeneous. We have studied a pediatric patients group in a short time period and this is the reason for a limited number of patients and not for other reasons related to the method. In addition MostCare-PRAM was compared with the reference method studying only single CI measurements. The comparison between different monitoring systems may give suitable findings when assessing single CI measurements, but significant differences between techniques might rise when comparing the tendencies of a number of CI values under different hemodynamic conditions (e.g., changes in volume loading, ventricular function, systemic vascular resistance). Furtheremore, the study was carried out under strict experimental conditions on children with congenital heart disease so our results to be extrapolated, are necessary on studies in larger populations with a prospective, interventional design warranted, also in conditions with cardiovascular instability.
CONCLUSION
In conclusion, in pediatric cardiac surgery, cardiac output monitoring remains very challenging despite the availability of many different technologies. Transpulmonary indicator dilution and arterial pulse contour analysis have been developed for the measurement of CO, in adult patients, however, they have a lot of limitations in pediatric cardiac surgery. Between the new technologies Mostcare-PRAM represents a new available noninvasive monitoring devices for CO measurement in children, through the analysis of the arterial pressure waveform, that does not require external calibration. To confirm the validation of this system, we conducted a prospective analysis of haemodynamic monitoring in pediatric patients admitted to cardiac surgery where we compared the MostCare-PRAM system with the Fick method to measure cardiac output also in patients with intracardiac shunts. This is the first validation study conducted on these kinds of patients where the MostCare-PRAM system was shown to estimate CI with a good level of agreement with the Fick method and this is the reason why this system should be used in clinical management to target CI values in pediatric cardiac surgery.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research was carried out after the formal approval of the local Ethics Committee of Azienda Ospedaliera di Padova and informed parental consent.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Informed parental consent was obtained for using the collected data.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


