All published articles of this journal are available on ScienceDirect.
Activated Factor Seven (aFVII) versus Aminocaproic Acid for Treatment of Traumatic Retro-Peritoneal Hematoma
Abstract
Introduction:
Off-labelled use of activated Factor VII (aFVII) in severe traumatic bleeding has been used as an alternative to aminocaproic.
Aim of Work:
The aim of this study is to compare the efficacy of aFVII with aminocaproic acid in the medical treatment of retroperitoneal bleeding, treatment of hypovolemic shock and preventing complications of massive blood transfusion.
Materials and Methods:
80 patients with traumatic retro-peritoneal hematoma were allocated into two groups of 40 patients each. Patients in Group A received aminocaproic acid, while patients of group B received aFVII.
The number of packed RBCs given to achieve the target Hb level and time to get to this target Hb level (>10 gm%) were recorded as indicators of control bleeding. Blood pressure, pulse, arterial blood gasses and urine output were recorded as indicators of successful treatment of hypovolemic shock. Hypoxic index, chest X-ray and coagulation profile were used as indicators for complications.
Results:
There was a significantly smaller number of packed RBCs given to patients of group B to achieve the target Hb level and this target Hb level was achieved in a shorter time. There was a significantly higher number of patients in group B compared to group A who had normal blood pressure, pulse and urine output, pH and bicarbonate concentration. There was a significantly smaller number of patients who developed DIC and TRALI in group B compared to group A.
Conclusion:
aFVII was more effective than aminocaproic acid and needed a shorter time to stop retroperitoneal bleeding, treat hypovolemic shock, restore adequate tissue perfusion and protect patients from complications of massive blood transfusion.
1. INTRODUCTION
Recombinant activated factor seven (aFVII) has been used safely and effectively in acquired hemophilia. Interest has been generated following several case reports of dramatic cessation of bleeding within minutes of administration of aFVII to severely traumatized patients with massive bleeding [1-5]. The success reported after off labeled use of aFVII in surgical life-threatening bleeding prompted us to study its use in retro-peritoneal bleeding in trauma patients. aFVII works through activation of the extrinsic pathway of the coagulation cascade which is triggered by intimal injury of the blood vessels and produced stable fibrin clot, characterized by being more stable and more difficult to be lysed by the fibrinolytic system than the clot developed by the intrinsic pathway of the coagulation cascade [6-8]. On the other hand, aminocaproic acid has enjoyed a long history of being a trustable safe drug. It has been used to stop severe life-threatening bleeding by stopping the fibrinolysis and protect the newly formed thrombus [9-11]. Both drugs can be used in a severe life-threatening retroperitoneal hematoma.
Traumatic retroperitoneal hematoma is a common complication of severe abdominal or/and pelvic injuries. Retro peritoneal space contains a number of visceral and vascular structures in the gastrointestinal, genitourinary, vascular, musculoskeletal and nervous systems [12]. It may be potentially responsible for the occurrence of traumatic retroperitoneal hematoma, and makes the diagnosis and treatment of such fatal lesion very difficult. A mortality rate of traumatic retroperitoneal hematoma is reported as high as 18-60% in published literature [13]. Early diagnosis and proper treatment are important to decrease the mortality of this life-threatening lesion. In recent years, despite the advances in surgical techniques, the diagnosis and treatment of traumatic retroperitoneal hematoma still remain challenging [14]. It is difficult to diagnose clinically and by routine screening ultrasound. Moreover, due to lack of the supporting tissue and the potential wide space of the retro-peritoneal cavity, this hematoma spreads and becomes life-threatening [15]. Since there is no clear surgical treatment for the retro-peritoneal hematoma, so both interventional radiological management and medications that support the coagulation profile of the patients are considered the cornerstone of the management. Unfortunately, interventional radiological management is not available in many centers beside it needs great experience. This put the pharmacological management with drugs supporting the patient's coagulation profile, the first line of management [14, 15].
2. AIM OF WORK
The aim of this work is to compare the efficacy of aFVII to aminocaproic acid in the medical treatment of retro-peritoneal bleeding, treatment of hypovolemic shock and prevention of complications from a massive blood transfusion.
3. MATERIALS AND METHODS
80 patients admitted to King Abdul-Aziz Specialist Hospital in Taif, KSA between May 2017 and December 2019 with polytrauma, and severe retroperitoneal bleeding diagnosed by abdominal computerized tomography, were eligible for this study.
King Abdel Aziz research and the ethical committee approved the project in compliance with the Helsinki Declaration. Written informed consent from the patient or from their 1st-degree relative was obtained if the patient was intubated. The primary outcome of this study was divided into 3 parts. The first part compares the ability of both drugs to stop retro-peritoneal bleeding efficiently and in a shorter time for these 2 measurable indicators selected in our study, the number of packed RBCs needed to achieve hemoglobin level (Hb) > 10 gm% and the time needed to achieve this target Hb. In the 2nd part, indicators are divided into clinical indicators (blood pressure, pulse and urine output) and laboratory indicators [arterial blood gases, especially PH, and bicarbonate concentration (HCO3)]. In the 3rd part indicators PT, PTT and platelet count were used as indicators for DIC and hypoxic index and chest x ray were used as an indicator for TRALI (transfusion-related acute lung injury).
Resuscitation was performed in all patients in both groups according to hospital protocol using crystalloid (normal saline) and colloids (plasma protein fraction, packed RBCs and fresh frozen plasma), until satisfactory stable vital data was achieved. All laboratory work samples were collected (CBC complete blood picture, blood chemistry included liver enzymes and kidney function tests, coagulation profile, arterial blood gasses).
Urine output was noted hourly.
Our study included a patient who received more than 50ml/kg blood during the first 8 hours after admission in order to achieve a hemoglobin level >10 gram %, with platelet count more than 50, 000/mm3 after resuscitation and temperature more than 36 degrees centigrade after warming, considered criteria needed to get the maximum effect from aFVII. Patients were randomized into 2 groups, 40 patients in each, using randomization numbers. Patients in group A received aminocaproic acid at a dose of 4 gram slowly intravenous infusion over 1 hour and continues slowly intravenous infusion 1 gram/ hour for 8 hours. While patients of group B received aFVII according to the following protocol,
First dose 200 microgram/kg slowly by intravenous infusion over 30 minutes. If patients were still bleeding, vital data were not stable and/or could not achieve and keep the target Hb (>10 gm%), another 2 doses of aFVII were given, each dose 100 microgram /kg 1 hour and 3 hours apart from the initial dose if needed. The exclusion criteria in our study, included patients who had a cardiac arrest before receiving aFVII, deeply comatose with the Glasgow coma scale (GCS) 3/15 with dilated and fixed pupils.
All patient in both groups were observed for 48 hours and the following indicators were recorded:
Indicators for the efficacy of stop bleeding (number of packed RBCs needed to keep hemoglobin level >10 gm% and time needed to reach this target Hb).
Indicators for the ability of both drugs to treat the hemorrhagic shock and restore tissue perfusion, and this assessed by clinical indicators blood pressure, pulse and urine output and laboratory indicators as arterial pH and bicarbonate concentration (HCO3). Indicators for the ability of both drugs to protect the patients with retro-peritoneal hematoma from complications of massive blood transfusion and in this, 2 complications were selected the disseminated intra-vascular coagulopathy (DIC) and PT, PTT and Platelets count were used as indicators and (TRALI) transfusion-related acute lung injury and the hypoxic index and chest X-ray were used as indicators. The duration of the study was for two days and the data recorded every 8 hours.
4. STATISTICAL ANALYSIS
Statistical analysis was done using Statistical Package for Social Sciences (SPSS/version 20) software.
The statistical test was used as follow:
Number and percent then for categorized parameters, Chi-square test was used. The level of significance was 0.05.
5. SAMPLE SIZE
The sample size was calculated based on a previous study on the uses of activated factor seven (aFVII) in severe bleeding in polytrauma patients, by using Med Calc statistical software.
Assuming area under ROC to be 0.80, an alpha of 0.05 and the power of study 90.0%. A minimum sample size required to evaluate the aFVII-in patients with retroperitoneal hematoma was 80 patients.
6. RESULTS
- In group A: no any patient had elevated cardiac enzymes [CPK MB or Troponin] or had ECG changes. 5 patients died on the 24th day from admission from TRAI, respiratory failure and multi org failure
- In group B: no any patient had elevated cardiac enzymes [CPK MB or Troponin] or had ECG changes. 1 patient died from chest infection [ventilator associated pneumonia] septic shock 2 weeks from admission.
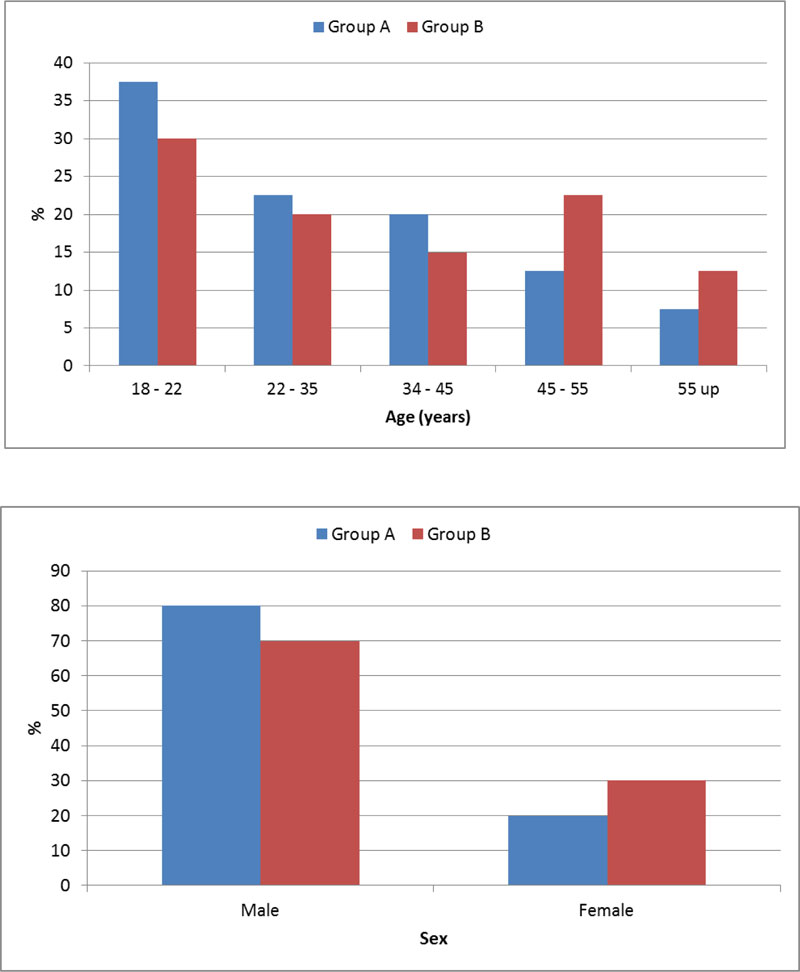
-Demographic data of patients in both groups illustrated in both Table 1 and Fig. (1).
And showed no significant different between patients in both groups as regard the age and the sex.
Indicators compared the ability of both drugs to stop the retroperitoneal bleeding in a faster time and more efficient way.
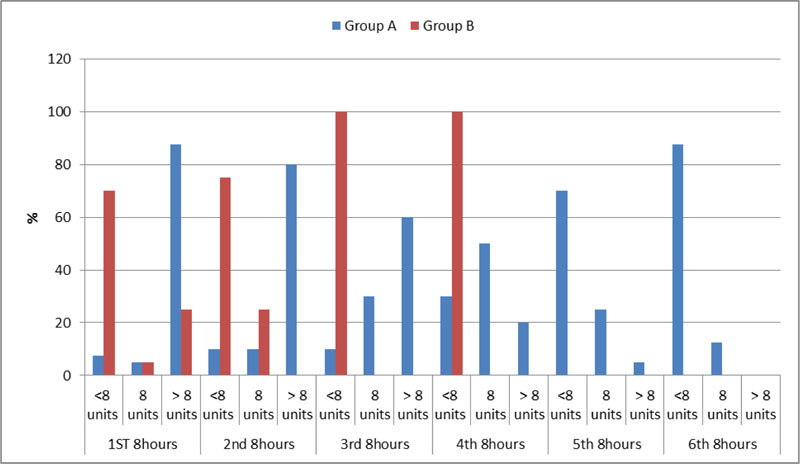
There was a significantly smaller number of packed RBCs units given to keep hemoglobin level >10 gm% in patients of group B compared to group A in all the 6 periods of the studied duration (2 days). In addition, Hb level was reached in a significantly shorter time in patients of group B compared to patients of group A. As in the 2nd 8 hours from given the aFVII, no any patient received more than 8 units packed RBCS to achieve the target Hb in group B compared to 32 patients received more than 8 units packed RBCS to achieve the target Hb in group A.
Number of packed RBCs received by all patients in both groups during the studied period illustrated in both Table 2 and Fig. (2).
| Age (years) | Group A | Group B | p | ||
|---|---|---|---|---|---|
| n=40 | % | n=40 | % | ||
| 18 – 22 | 15 | 37.5 | 12 | 30.0 | 0.136 |
| 22 – 35 | 9 | 22.5 | 8 | 20.0 | |
| 34 – 45 | 8 | 20.0 | 6 | 15.0 | |
| 45 – 55 | 5 | 12.5 | 9 | 22.5 | |
| 55 up | 3 | 7.5 | 5 | 12.5 | |
| Sex | 0.336 | ||||
| Male | 32 | 80.0 | 28 | 70.0 | |
| Female | 8 | 20.0 | 12 | 30.0 | |
| Units of blood | Group A | Group B | P value | |||
|---|---|---|---|---|---|---|
| n=40 | % | n=40 | % | |||
| 1ST 8hours | <8 units 8 units > 8 units |
3 2 35 |
7.5 5 87.5 |
28 2 10 |
70.0 5.0 25.0 |
0.001* |
| 2nd 8hours | <8 units 8 units > 8 units |
4 4 32 |
10.0 10.0 80.0 |
30 10 0 |
75.0 25.0 0.0 |
0.001* |
| 3rd 8hours | <8 units 8 units > 8 units |
4 12 24 |
10.0 30.0 60.0 |
40 0 0 |
100.0 0.0 0.0 |
0.001* |
| 4th 8hours | <8 units 8 units > 8 units |
12 20 8 |
30.0 50.0 20.0 |
40 0 0 |
100.0 0.0 0.0 |
0.001* |
| 5th 8hours | <8 units 8 units > 8 units |
28 10 2 |
70.0 25.0 5.0 |
0 0 0 |
0.0 0.0 0.0 |
0.0012* |
| 6th 8hours | <8 units 8 units > 8 units |
35 5 0 |
87.5 12.5 0.0 |
0 0 0 |
0.0 0.0 0.0 |
0.0011* |
Indicators compared the ability of both drugs to treat hypovolemic shock and restore adequate tissue perfusion
The number of patients showed improvement in the hemodynamic parameters (return both BP and pulse to their normal measure) and showed normal urine output, normal PH and normal bicarbonate concentration significantly higher in group B compared to group A in the studied period.
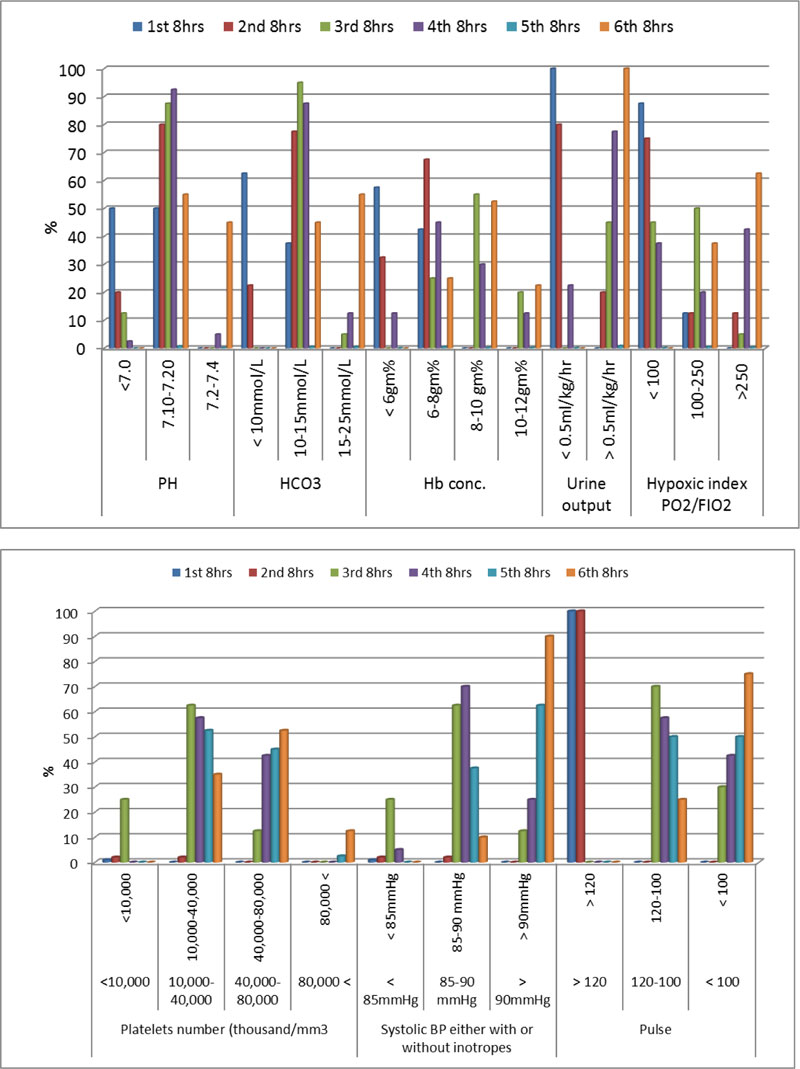
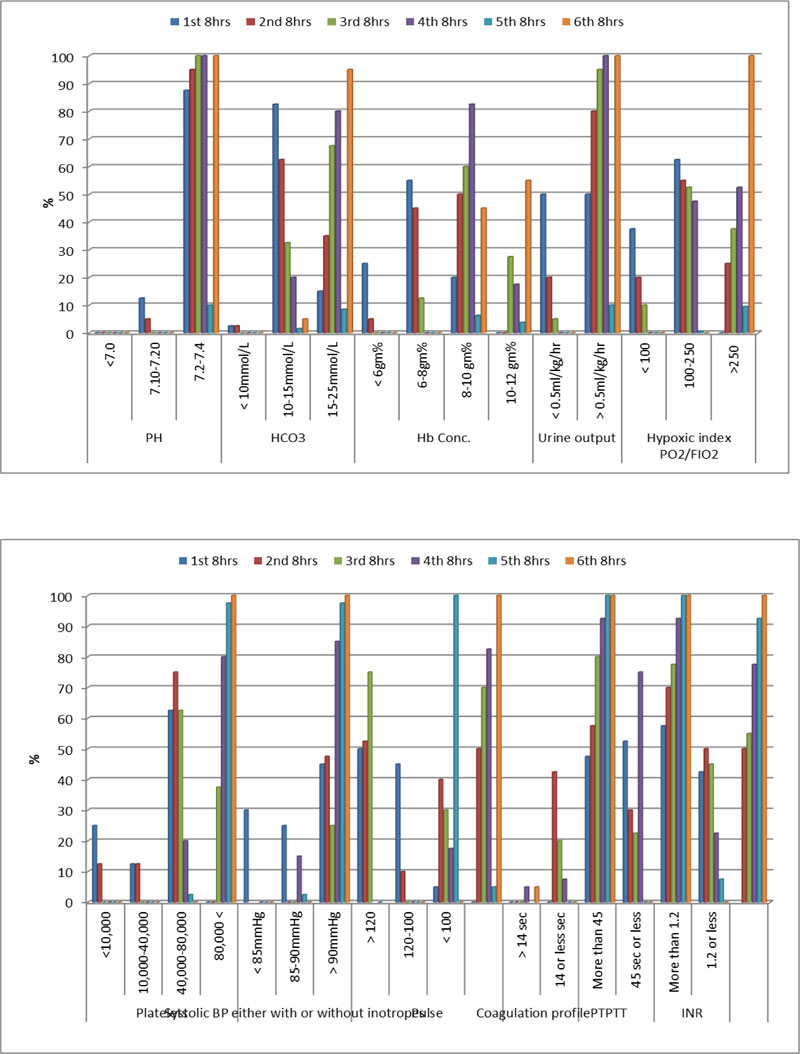
Hemodynamic and laboratory data of patients in group A during the studied period illustrated in both Table 3 and Fig. (3). While hemodynamic and laboratory data of patients in group B during the studied period illustrated in both Table 4 and Fig. (4).
| ABG Group A | 1st 8hrs | 2nd 8hrs | 3rd 8hrs | 4th 8hrs | 5th 8hrs | 6th 8hrs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n=40 | % | n=40 | % | n=40 | % | n=40 | % | n=40 | % | n=40 | % | ||
| PH | <7.0 | 20 | 50 | 8 | 20 | 5 | 12.5 | 1 | 2.5 | 0 | 0 | 0 | 0 |
| 7.10-7.20 | 20 | 50 | 32 | 80 | 35 | 87.5 | 37 | 92.5 | 31 | 0.775 | 22 | 55 | |
| 7.21-7.4 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 5 | 9 | 0.225 | 18 | 45 | |
| HCO3 | < 10mmol/L | 25 | 62.5 | 9 | 22.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10-15mmol/L | 15 | 37.5 | 31 | 77.5 | 38 | 95 | 35 | 87.5 | 21 | 52.5 | 18 | 45 | |
| 16-25mmol/L | 0 | 0 | 0 | 0 | 2 | 5 | 5 | 12.5 | 19 | 47.5 | 22 | 55 | |
| Hb conc. | < 6gm% | 23 | 57.5 | 13 | 32.5 | 10 | 0 | 5 | 12.5 | 2 | 5 | 0 | 0 |
| 6-8gm% | 17 | 42.5 | 27 | 67.5 | 22 | 25 | 18 | 45 | 18 | 45 | 10 | 25 | |
| 8-10 gm% | 0 | 0 | 0 | 0 | 8 | 55 | 12 | 30 | 11 | 27.5 | 21 | 52.5 | |
| 10-12gm% | 0 | 0 | 0 | 0 | 0 | 20 | 5 | 12.5 | 9 | 22.5 | 9 | 22.5 | |
| Urine output | < 0.5ml/kg/hr. | 40 | 100 | 32 | 80 | 18 | 0 | 9 | 22.5 | 5 | 12.5 | 0 | 0 |
| > 0.5ml/kg/hr. | 0 | 0 | 8 | 20 | 22 | 45 | 31 | 77.5 | 35 | 87.5 | 40 | 100 | |
| Hypoxic index PO2/FIO2 | < 100 100-250 >250 |
35 5 0 |
87.5 12.5 0 |
30 5 5 |
75 12.5 12.5 |
18 20 2 |
45 50 5 |
15 17 8 |
37.5 42.5 20 |
5 20 15 |
12.5 5 37.5 |
0 15 25 |
0 37.5 62.5 |
| Cardiac enzymes ECG charges | Within normal in all over the duration of the study | ||||||||||||
| Platelets number (thousand/mm3) | <10,000 | 40 | 100 | 20 | 50.0 | 25 | 62.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10,000-40,000 | 0 | 0 | 20 | 50.0 | 10 | 25.0 | 23 | 57.5 | 21 | 52.5 | 14 | 35 | |
| 40,000-80,000 | 0 | 0 | 0 | 0 | 5 | 12.5 | 17 | 42.5 | 18 | 45 | 21 | 52.5 | |
| 80,000 < | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2.5 | 5 | 12.5 | |
| Systolic BP either with or without inotropes | < 85mmHg 85-90 mmHg > 90mmHg |
40 0 0 |
100 0 0 |
20 20 0 |
50.0 50.0 0.0 |
10 25 5 |
25.0 62.5 12.5 |
2 28 10 |
5 70 25 |
0 15 25 |
0 37.5 62.5 |
0 4 36 |
0 10 90 |
| Pulse (beat/ min) |
> 120 120-100 < 100 |
40 0 0 |
100 0 0 |
40 0 0 |
100 0 0 |
0 28 12 |
0 70 30 |
0 23 17 |
0 57.5 42.5 |
0 20 20 |
0 50 50 |
0 10 30 |
0 25 75 |
| Chest x-ray Which show Evidence of bilateral tinny lung infiltrate Indicate (TRALI) | 0 | 0 | 2 | 5 | 6 | 15 | 8 | 20 | 8 | 20 | 8 | 20 | |
|
Coagulation profile PT |
> 14 sec 14 or less sec |
40 0 |
100 0.0 |
32 8 |
80 20 |
28 12 |
70 30 |
25 15 |
62.5 37.5 |
20 20 |
50 50 |
18 22 |
45 55 |
| PTT | More than 45 45 sec or less |
40 0 |
100 0.0 |
35 5 |
87.5 12.5 |
30 10 |
75 25 |
28 12 |
70 30 |
24 16 |
60 40 |
17 23 |
42.5 57.5 |
| INR | More than 1.2 1.2 or less |
40 0 |
100 0.0 |
40 0 |
100 0.0 |
31 9 |
77.5 22.5 |
25 15 |
62.5 37.5 |
23 17 |
57.5 42.5 |
20 20 |
50 50 |
| ABG Group B | 1st 8hrs |
2nd 8hrs | 3rd 8hrs |
4th 8hrs |
5th 8hrs |
6th 8hrs |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n=40 | % | n=40 | % | n=40 | % | n=40 | % | n=40 | % | n=40 | % | ||
| PH | <7.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7.10-7.20 | 5 | 12.5 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 7.21-7.4 | 35 | 87.5 | 38 | 95 | 40 | 100 | 40 | 100 | 40 | 100 | 40 | 100 | |
| HCO3 | < 10mmol/L | 1 | 2.5 | 1 | 2.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10-15mmol/L | 33 | 82.5 | 25 | 62.5 | 13 | 32.5 | 8 | 20 | 6 | 15 | 2 | 5 | |
| 16-25mmol/L | 6 | 15 | 14 | 35 | 27 | 67.5 | 32 | 80 | 34 | 85 | 38 | 95 | |
| Hb Conc. | < 6gm% | 10 | 25 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6-8gm% | 22 | 55 | 18 | 45 | 5 | 12.5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 8-10 gm% | 8 | 20 | 20 | 50 | 24 | 60 | 33 | 82.5 | 25 | 62.5 | 18 | 45 | |
| 10-12 gm% | 0 | 0 | 0 | 0 | 11 | 27.5 | 7 | 17.5 | 15 | 37.5 | 22 | 55 | |
| Urine output | < 0.5ml/kg/hr | 20 | 50 | 8 | 20 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| > 0.5ml/kg/hr | 20 | 50 | 32 | 80 | 38 | 95 | 40 | 100 | 40 | 10 | 40 | 100 | |
| Hypoxic index PO2/FIO2 | < 100 100-250 >250 |
15 25 0 |
37.5 62.5 0 |
8 22 10 |
20 55 25 |
4 21 15 |
10 52.5 37.5 |
0 19 21 |
0 47.5 52.5 |
0 2 38 |
0 5 95 |
0 0 40 |
0 0 100 |
| Cardiac enzymes ECG charges | Within normal in all | ||||||||||||
| Platelets Thousands/mm3 |
<10,000 | 10 | 25 | 5 | 12.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10,000-40,000 | 5 | 12.5 | 5 | 12.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 40,000-80,000 | 25 | 62.5 | 30 | 75 | 25 | 62.5 | 8 | 20 | 1 | 2.5 | 0 | 0 | |
| 80,000 < | 0 | 0 | 0 | 0 | 15 | 37.5 | 32 | 80 | 39 | 97.5 | 40 | 100 | |
| Systolic BP either with or without inotropes | < 85mmHg 85-90mmHg > 90mmHg |
12 10 18 |
30 25 45 |
0 19 21 |
0 47.5 52.5 |
0 10 30 |
0 25 75 |
0 6 34 |
0 15 85 |
0 1 39 |
0 2.5 97.5 |
0 0 40 |
0 0 100 |
| Pulse (beat/min) |
> 120 120-100 < 100 |
20 18 2 |
50 45 5 |
4 16 20 |
10 40 50 |
0 12 28 |
0 30 70 |
0 7 33 |
0 17.5 82.5 |
0 0 40 |
0 0 100 |
0 0 40 |
0 0 100 |
| Chest x-ray (picture of TRALI) | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 5 | 2 | 5 | 2 | 5 | |
|
Coagulation profile PT |
> 14 sec 14 or less sec |
26 14 |
65.0 35.0 |
17 23 |
42.5 57.5 |
8 32 |
20 80 |
3 37 |
7.5 92.5 |
0 40 |
0 100 |
0 40 |
0 100 |
| PTT | More than 45 45 sec or less |
19 21 |
47.5 52.5 |
12 28 |
30 70 |
9 31 |
22.5 77.5 |
3 37 |
7.5 92.5 |
0 40 |
0 100 |
0 40 |
0 100 |
| INR | More than 1.2 1.2 or less |
23 17 |
57.5 42.5 |
20 20 |
50 50 |
18 22 |
45 55 |
9 31 |
22.5 77.5 |
3 37 |
7.5 92.5 |
0 40 |
0 100 |
| 3rd 8hrs | p | 6th 8hrs | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A | Group B | Group A | Group B | ||||||||
| n=40 | % | n=40 | % | n=40 | % | n=40 | % | ||||
| PH | <7.0 | 5 | 12.5 | 0 | 0 | 0.001* | 0 | 0 | 0 | 0 | 0.016* |
| 7.10-7.20 | 35 | 87.5 | 0 | 0 | 22 | 55 | 0 | 0 | |||
| 7.21-7.4 | 0 | 0 | 40 | 100 | 18 | 45 | 40 | 100 | |||
| HCO3 | < 10mmol/L | 0 | 0 | 0 | 0 | 0.013* | 0 | 0 | 0 | 0 | 0.011* |
| 10-15mmol/L | 38 | 95 | 13 | 32.5 | 18 | 45 | 2 | 5 | |||
| 16-25mmol/L | 2 | 5 | 27 | 67.5 | 22 | 55 | 38 | 95 | |||
| Hb Conc. | < 6gm% | 10 | 0 | 0 | 0 | 0.226 | 0 | 0 | 0 | 0 | 0.042* |
| 6-8gm% | 22 | 25 | 5 | 12.5 | 10 | 25 | 0 | 0 | |||
| 8-10 gm% | 8 | 55 | 24 | 60 | 21 | 52.5 | 18 | 45 | |||
| 10-12 gm% | 0 | 20 | 11 | 27.5 | 9 | 22.5 | 22 | 55 | |||
| Urine output | < 0.5ml/kg/hr. | 18 | 0 | 2 | 5 | 0.412 | 0 | 0 | 0 | 0 | - |
| > 0.5ml/kg/hr. | 22 | 45 | 38 | 95 | 40 | 100 | 40 | 100 | |||
| Hypoxic index PO2/FIO2 | < 100 100-250 >250 |
18 20 2 |
45 50 5 |
4 21 15 |
10 52.5 37.5 |
0.033* | 0 15 25 |
0 37.5 62.5 |
0 0 40 |
0 0 100 |
0.031 |
| Platelets Thousands/mm3 |
<10,000 | 25 | 62.5 | 0 | 0 | 0.001* | 0 | 0 | 0 | 0 | 0.001* |
| 10,000-40,000 | 10 | 25.0 | 0 | 0 | 14 | 35 | 0 | 0 | |||
| 40,000-80,000 | 5 | 12.5 | 25 | 62.5 | 21 | 52.5 | 0 | 0 | |||
| 80,000 < | 0 | 0 | 15 | 37.5 | 5 | 12.5 | 40 | 100 | |||
| Systolic BP either with or without inotropes | < 85mmHg 85-90mmHg > 90mmHg |
10 25 5 |
25.0 62.5 12.5 |
0 10 30 |
0 25 75 |
0.021* | 0 4 36 |
0 10 90 |
0 0 40 |
0 0 100 |
0.23 |
| Pulse (beat/min) |
> 120 120-100 < 100 |
0 28 12 |
0 70 30 |
0 12 28 |
0 30 70 |
0.001* | 0 10 30 |
0 25 75 |
0 0 40 |
0 0 100 |
0.045* |
| Chest x-ray (picture of TRALI) | 6 | 15 | 0 | 0 | 0.041* | 8 | 20 | 2 | 5 | 0.044* | |
| Coagulation profile | |||||||||||
| PT | > 14 sec 14 or less sec |
28 12 |
70 30 |
8 32 |
20 80 |
0.002* | 18 22 |
45 55 |
0 40 |
0 100 |
0.021* |
| PTT | More than 45 45 sec or less |
30 10 |
75 25 |
9 31 |
22.5 77.5 |
0.001* | 17 23 |
42.5 57.5 |
0 40 |
0 100 |
0.010* |
| INR | More than 1.2 1.2 or less |
31 9 |
77.5 22.5 |
18 22 |
45 55 |
0.015* | 20 20 |
50 50 |
0 40 |
0 100 |
0.01* |
Table 5 Compared both laboratory and hemodynamic data of all patients in both groups during the studied period.
Indicators compared the ability of both drugs to protect the patients from complications of massive blood transfusion
A number of patients showed normal coagulation profile, normal hypoxic index, and normal chest X-ray (without parenchymatous lung infiltrate, suggesting TRALI) significantly higher in group B compared to group A in the studied period.
Chest X ray suggesting picture of TRALI and deranged coagulation profile suggesting DIC between patients of group A in the studied period illustrated in both Table 3 and Fig. (3). While same clinical data between patients of group B in the studied period illustrated in both Table 4 and Fig. (4).
7. DISCUSSION
Aminocaproic acid is an antifibrinolytic drug. It was first tested in humans by Japanese researcher Okamoto in 1950. It is used in clinical practice in severe traumatic bleeding. Since then, it is a reliable antifibrinolytic agent. Our study compared aminocaproic acid with off-labelled indication of aFVII in a severe retroperitoneal hematoma.
The off labeled indication of aFVII has been studied by many authors, especially its use in trauma patients with severe bleeding. In our study, there was a significantly smaller number of packed RBCs units given to keep hemoglobin level >10 gm% in patients of group B compared to group A in all the 6 periods of the studied duration (2 days) that Hb level reached in significantly shorter time in patients of group B compared to patients of group A. As in the 2nd period, 8 hours after given the aFVII, no patient received more than 8 units packed RBCS to achieve the target Hb in group B compared to 32 patients in group A, who received more than 8 units packed RBCS to achieve the target Hb. This can be explained by the unique action of aFVII mentioned before on the injured area and the more stable blood clot formed by it. At pharmacological concentration of aFVII, the thrombin produced is more resistant to endogenous thrombolysis and therefore leads to more stable clots, which is produced in minutes after its administration [15-18].
There was a significantly higher number of patients in group B compared to group A who showed both normal clinical and normal laboratory data, which suggested complete resolution of hypovolemic shock (normal BP, normal pulse, normal pH, and normal HCO3 level), A non-significant higher number of patients showed normal urine output (> 0.5ml/kg/hr.) in group B compared to group A. This was also achieved in a significantly shorter time after massive retroperitoneal hemorrhage. This can be explained by the well-known physiological rule in any life-threatening bleeding that efficient and complete cessation of blood loss in short duration enables the compensatory mechanism to restore tissue perfusion and stop the cascade of tissue injury from both hypotension and severe anemia due to blood loss. The best clinical indicator for this was the return back of the urine output to normal, while the best laboratory indicator for this was the return back of normal PH and bicarbonate level to normal. This reflected normal aerobic cellular metabolism and proved that lactic acidosis was completely washed from restored tissue perfusion after prolonged tissue hypoxia and anaerobic cellular metabolism. In hemorrhagic shock, the time is very important as the faster you control the bleeding, the better prognosis.
There was a significantly higher number of patients in group B compared to group A who showed normal coagulation profile and normal chest Xray (without parenchymatous lung infiltrate suggestive of TRALI). This demonstrated that aFVII may be more efficient in the protection of the patient with life-threatening hemorrhage from massive blood transfusion complications (DIC and TRALI). One of the most common complications of massive blood transfusion is coagulopathy as the transfused blood contains no clotting factors combined with the release of tissue factor into the blood from the injured traumatized retroperitoneal tissues which can cause DIC due to activation of the extrinsic pathway of coagulation. aFVII efficiently and rapidly controlled bleeding, limiting the need for blood transfusion. The same mechanism can be applied in the protection of patients in group B from TRALI as it is one of the most common complications of massive blood transfusion through immunological mechanisms. As the transfused blood work as an antigen, which reacts with patient's antibodies and fixes the complement end by destroying alveoli-capillary membrane and pouring of transudate inside the alveoli and give picture like ARDS (acute respiratory distress syndrome). The more the blood transfusion, the more antigen in the blood, the more aggressive immunological reaction and the more destruction of the lung tissue.
In this study, we gave aFVII to patients with platelet count more than 50, 000/mm3 and temperature more than 36 degrees centigrade. The activity of aFVII is markedly reduced (more than 20%) if the temperature less than 33 degrees centigrade and the platelets are less than 50,000/mm3 [8, 17, 21].
Our study supports many studies conducted on aFVII in this field.
Martinowitz et al., in 2001 [16], did a retrospective single-center study in penetrating or blunt trauma in seven patients with severe bleeding and found significant improvement in the coagulation profile and stop of bleeding in patients given aFVII.
Dutton et al., in 2004 [17], used aFVII on 46 patients with severe trauma and bleeding. He found that 28patients from 46 given aFVII had normal coagulation factors and had marked the cessation of bleeding with a significant reduction in the number of packed red blood cells needed.
Geeraedts et al., in 2005 [18], did nearly the same study in bleeding blunt trauma and had the same result but cessation of bleeding and normalization of coagulation factors achieved in all his patients with 100% success.
Cameron et al., in 2007 [19], published his experience in the use of aFVII in trauma patients in Australia and New Zealand and proved that there was a significant decrease in bleeding in 59% of trauma patients who had severe bleeding and a significant reduction in blood transfusion requirements by the same percent.
Spinella et al., in 2008 [20], conducted another study on 124 patients with severe trauma and massive bleeding. In this study, 49 patients were given aFVII and 75 patients were in the control group and he found a significant reduction in the mortality rate between patients given aFVII compared to patients in the control group.
Many studies also discussed the maximum activity of the aFVII and the factors affecting his activity in vitro because as we mentioned before, it is an expensive drug and maximum activity should be getting from it when administered, especially when given in life-threatening bleeding because it considered the last bullet in the piston.
Dutton et al., 2004 [17], published a marked reduction in the activity of aFVII if used on low arterial PH (7.02 versus 7.29).
Meng et al., 2003 [21], prove in his study that activity of aFVII/ tissue factor complex was reduced by 20% when aFVII is used in temperature 33 degrees Centigrade and in acidosis.
On the other hand, some authors and clinicians still did not support the off labeled indication of aFVII in severe bleeding due to trauma because it is an expensive drug and can have thromboembolic complications.
Kathryn et al. [22], concluded that most of the reported thromboembolic events followed the use of aFVII for unlabeled indications that occurred in arterial and venous systems and often resulting in serious morbidity and mortality. He advises more randomized studies to determine its efficacy and safety.
The limitation of this study was the small sample size and our study concentrates only on two complications of massive blood transfusion (DIC, and TRALI). We found aFVII very effective in hemostasis, yet it is very expensive and this limits its use on a larger scale. We did not find any thromboembolic events between patients of group B but still more research work needed to study its safety if used on a large scale. Also, studies should be done to standardize a fixed dose and regimen of aFVII in traumatic bleeding because aFVII given in different doses and different regimens in the research work, and up till now, no agreement between authors on specific dose or regimen in severe life-threatening hemorrhage.
CONCLUSION
aFVII was more effective than aminocaproic acid and required shorter time to stop retroperitoneal bleeding, treat hypovolemic shock, restore adequate tissue perfusion and protect patients from complications of massive blood transfusion.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the research and ethical committee of King Abdel Aziz University, Saudi Arabia.
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent from the patient or from their 1st-degree relative was obtained if the patient was intubated.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


