Comparison of the Frequency of Gastrointestinal Bleeding Complications Resulting from the use of Ketorolac after Gastrointestinal Cancer Surgery with or without Gastric Ulcer Prophylaxis - A Case Control Study
Abstract
Background:
Gastrointestinal (GI) bleeding after GI cancer surgery is not very common, but the use of NSAIDs such as Ketorolac can aggravate it, and if not controlled properly, it may be life-threatening. Therefore, an NSAID/PPI combination (ketorolac and Pantoprazole) that reduces the adverse effect of ketorolac on GI bleeding can be very important.
Aim:
The aim of this observational study is to compare the frequency of GI bleeding complications resulting from the use of Ketorolac after GI cancer surgery with or without gastric ulcer prophylaxis (Pantoprazole).
Methods:
In this retrospective case-control study, the medical files of adult patients aged 18-60 years undergoing GI cancer surgery referred to 3 hospitals in Iran in 2022 were reviewed. The case group consisted of patients who received ketorolac (30 mg every 8 hours, intravenously) with preventive Pantoprazole (40 mg daily). The control group consisted of patients who only received ketorolac (30 mg every 8 hours, intravenously). Patients were matched in groups based on demographic and clinical variables. Outcomes, including GI bleeding (melena, ...), length of hospital and ICU stay, receiving packed cells, intubation, hematocrit and hemoglobin, were compared between the groups.
Results:
Two groups were matched in terms of age, gender, comorbidities, type of surgery, duration of surgery (hours), and surgical bleeding (ml) (P>0.05). Examination of clinical outcomes showed that GI bleeding complications were not significantly different in the two groups. Although in the case group that received ketorolac and Pantoprazole combination, GI bleeding complications were reported in a smaller number of people. The hospital stay (days) was significantly lower in the case group than in the control group. The ICU stay (hours), packed cells, intubation, hematocrit, and hemoglobin were not significantly different between the two groups.
Conclusion:
The findings of the current study showed that the administration of Pantoprazole plus ketorolac might be effective in controlling bleeding in GI cancer surgery patients, which, of course, requires detailed and multicenter interventional studies.
1. INTRODUCTION
Currently, cancer is known as the third cause of death in Iran. In most of the studies conducted in Iran, GI cancers are among the most prevalent cancers, and stomach cancer is the most common cause of cancer mortality in Iran [1-3]. One of the GI cancers treatment is surgery along with chemotherapy and radiotherapy, which are associated with some complications like pain. Among the widely used medications for pain control are NSAIDs (Nonsteroidal Anti-inflammatory Drugs) [4]. Ketorolac Tromethamine is an NSAID with a short-term treatment of moderate to severe pain, one of the strong inhibitors of the cyclooxygenase enzyme, which inhibits prostaglandins, and it is used more as an analgesic than an anti-inflammatory medication [5]. This medication reduces the need to use opioids. However, despite its various usefulness and good efficiency, its use can be associated with some complications. In acute use, it causes GI (GI) bleeding with the effect on platelets, and in long-term use, it causes destruction of the mucosa with the effect on gastric mucosa and also reduces kidney function [6]. Clinically, the rate of upper GI bleeding in NSAID users is estimated at 1-2.5 per 100 people per year [7]. Available evidence shows that NSAIDs, along with causing bleeding from the upper GI tract, also raise the risk of bleeding from the lower GI tract to the same extent [8].
Studies have shown that the simultaneous use of GI prophylaxis agents in continuous NSAID users can reduce the risk of GI bleeding [9, 10]. Therefore, many scientific guidelines recommend that GI prophylaxis be used in NSAID users at high risk of GI bleeding [11]. Currently, the treatment and prevention of NSAID-related lower GI bleeding are challenging; because the possible pathogenic mechanisms are diverse and not well-defined. GI prophylaxis mainly includes acid suppressants such as histamine-2 receptor antagonists (H2RA] and proton pump inhibitors (PPIs), which primarily have a protective effect against upper GI damage. GI prophylaxis in addition to acid suppressants, including misoprostol and rebamipide, have different mechanisms to protect the GI tract. Moreover, some investigations have demonstrated that misoprostol and rebamipide are effective against NSAID-related GI damage; however, the mechanisms are still unclear [12, 13].
In an experimental study in Egypt in 2015, it was shown that the simultaneous use of NSAID and PPI combination is the best agent for the treatment and prevention of GI bleeding damage caused by the use of NSAIDs [14]. Another investigation of 84 aspirin users demonstrated that PPIs were better than placebo in treating aspirin-induced small bowel ulcers [15]. Similarly, other prospective surveys showed that PPIs were effective for the treatment of small bowel ulcers in 104 patients taking low-dose aspirin or an NSAID [16].
Although, in general, GI bleeding after GI cancer surgery is rare, the use of NSAIDs can aggravate it. If these bleedings are not properly controlled, they can be life-threatening. Therefore, combination therapy that reduces the adverse effect of ketorolac on GI bleeding can be very important. A suitable NSAID/PPIs combination is the ketorolac and Pantoprazole combination. It should be noted that the NSAID/PPIs combination for treating the symptoms of osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis and reducing the risk of stomach ulcers in high-risk patients has been approved by the FDA [17]. Pantoprazole, with the chemical formula of C16H15F2N3O4S, is a proton pump inhibitor that leads to a decrease in gastric acid secretion. It suppresses gastric acid secretion by irreversibly inhibiting hydrogen potassium ATPase in gastric parietal cells [18].
In the current observational study, the effect of the ketorolac and Pantoprazole combination in patients with GI cancers to control GI bleeding complications caused by the administration of ketorolac in the acute situation after surgery was investigated.
2. METHODS
2.1. Study Setting
This retrospective, observational, case-control study was conducted on patients undergoing surgery for GI cancers in the adult age group of 18-60 years old who were referred to 3 hospitals in Iran in 2022.
2.2. Population
The medical files of patients who underwent surgery for GI cancers and received ketorolac or ketorolac and Pantoprazole combination for pain relief were included in the study considering the inclusion and exclusion criteria.
2.3. Inclusion Criteria
Adult patients with GI cancers aged 18-60 years, GCS equal to 15, minimum one-day stay in ICU, no relative and absolute contraindications to receiving ketorolac or pantoprazole, complete medical file.
2.4. Exclusion Criteria
Incomplete medical files, children and the elderly, history of or suffering from stomach ulcers, active GI bleeding, use of NSAIDs and aspirin, previous history of GI surgery, acute or chronic kidney and liver failure, sensitivity, and any contraindications to NSAIDs, pregnancy or suspicion of pregnancy, history of alcohol or drug addiction, known allergies, history of mental illness and depression and recent use of sedatives or antipsychotics and use of calcium channel blockers, history of heart disease, seizures and history of hypotension, instability of the patient's clinical conditions, patients with uncontrolled pain and received other post-operative analgesics in addition to ketorolac.
2.5. Clinical Diagnosis of GI Cancers
The clinical diagnosis of GI cancer was made based on the diagnosis of the specialist physicians and the medical files of the patients.
2.6. Study Procedure and Data Gathering
At first, the necessary coordination was done with the management of the hospitals and legal permission was obtained for the researchers to access the medical files of the patients. Data were extracted from the medical files and recorded in the initial checklist. A trained team of researchers independently reviewed and cross-checked the data. If the core data was not available, the physicians responsible for treating the patients were contacted for clarification. Incomplete files were also excluded from the study.
Medical files of patients (male and female, age 18-60 years old) who underwent surgery for GI cancers and received ketorolac or ketorolac and Pantoprazole combination were enrolled. Demographic data (age and sex) and clinical information (comorbidities, type of surgery due to GI cancers) were extracted from medical files.
According to the medical files, the case group included patients who received ketorolac (30 mg every 8 hours, intravenously) with a preventive Pantoprazole (40 mg daily) combination, and the control group included patients who received ketorolac (30 mg every 8 hours, intravenously).
2.7. Groups Matching
It was tried to match the patients in the two groups in terms of age, gender, comorbidities, type of surgery, duration of surgery (hours), and surgery bleeding (ml).
2.8. Clinical Outcomes
The ICU (hours) and hospital (days) stay, intubation (hours), receiving packed cells, GI bleeding complications (upper bleeding, melena, rectorate, occult blood, wound bleeding, vomiting blood, blood return from the nasogastric tube), hemoglobin level (first 5 days), hematocrit level (first 5 days) after the surgery were extracted from each patient's medical file.
2.9. Possible Side Effects of the Medication
Signs of an allergic reaction (rash, hives, trouble breathing, itching, chest tightness, or swelling of the mouth, face, or lips), hot flashes or fainting, dizziness, heart arrhythmias, muscle paralysis or muscle weakness, severe sleepiness, and sweating were extracted from the patient's file.
2.10. Sampling Method and Sample Size Calculation
In the present study, an available sampling method was used, and according to the inclusion and exclusion criteria, all medical files of patients in 3 hospitals were enrolled.
2.11. Data Analysis
The continuous variables were expressed as the mean ± SD, and the categorical variables were presented as a percentage. Mann–Whitney U test and independent t-test were used to compare data between the two groups. All statistical analyses were performed with SPSS (version 16.0, SPSS Inc, Chicago, IL, USA). A “P-value” less than 0.05 was considered significant.
2.12. Ethical Considerations
The study was conducted in accordance with the Declaration of Helsinki. Institutional Review Board approval (Code: IR.MUMS.REC.1386.085) was obtained. The present study did not interfere with the process of diagnosis and treatment of patients. All data were extracted from the patients' medical records and kept confidential.
3. RESULTS
In the present case-control study, the medical files of 137 patients in the age group of 18-60 years who underwent GI cancer surgery were enrolled, 47 of whom were in the case group (ketorolac and Pantoprazole combination) and 90 people were included in the control group (ketorolac).
3.1. Demographic and Clinical Data
There was no significant difference between the mean age of patients, gender distribution and comorbidities such as diabetes, blood pressure, etc., in the two groups. About half of the patients in each group underwent upper GI surgery. The duration of surgery (hours) and surgery bleeding (ml) were not significantly different between the two groups (Table 1).
3.2. Clinical Outcomes
A small number of patients received packed cells during the operation, and no significant difference was recorded between the two groups. The hospital stay (days) was significantly lower in the case group than in the control group. The ICU stay (hours) was not significantly different between the two groups. The intubation and duration of intubation (minutes) were not significantly different between the groups. GI bleeding was not significantly different between the two groups. Although in the case group, GI bleeding was reported in a smaller number of people (Table 2).
| - | - | Case Group (n=47) | Control Group (n=90) | P value |
| Age (years) | Mean ± SD | 46.9 ± 11.8 | 47.4 ± 13.6 | 0.83 |
| - | Min-Max | 24-60 | 18-59 | - |
| Gender | Man, % | 16 (34 %) | 41 (45.5 %) | 0.19 |
| - | Female, % | 31 (66 %) | 49 (54.5 %) | - |
| Comorbidities | N (%) | 18 (38.3 %) | 37 (41.1 %) | 0.75 |
| Type of surgery | Upper GI system | 24 (51 %) | 43 (47.8 %) | 0.72 |
| - | Lower GI tract | 10 (21.3 %) | 22 (24.4 %) | - |
| - | Liver and pancreas | 5 (10.6 %) | 8 (8.9 %) | - |
| - | Other | 8 (17.1 %) | 17 (18.9 %) | - |
| Operation time (hours) | Mean ± SD | 5.7 ± 2.3 | 6 ± 1.7 | 0.86 |
| - | Min-Max | 1.4 - 9.2 | 3.9 - 10.7 | - |
| Intraoperative bleeding (ml) | Mean ± SD | 114.4 ± 173.1 | 159 ± 181.3 | 0.16 |
| - | Min-Max | 0-500 | 0-800 | - |
| - | - | Case group (n=47) | Control group (n=90) | P value |
| Receive packed cells | N (%) | 11 (23.4 %) | 24 (26.7 %) | 0.67 |
| Hospital stay (days) | Mean ± SD | 9.6 ± 4.1 | 13.5 ± 5.9 | 0.0001 |
| - | Min-Max | 15-Jun | 17-Jul | - |
| ICU stay (hours) | Mean ± SD | 49.5 ± 24.9 | 55 ± 23.1 | 0.2 |
| - | Min-Max | 24-96 | 24-72 | - |
| Intubation | N (%) | 18 (38.3 %) | 36 (40 %) | 0.84 |
| Intubation time (min) | Mean ± SD | 10.3 ± 10.4 | 12.6 ± 10.3 | 0.21 |
| - | Min-Max | 0-25 | 0-24 | - |
| GI bleeding * | N (%) | 8 (17 %) | 22 (24.4 %) | 0.32 |
| Day | Case group (n=47) | Control group (n=90) | P value |
| First | 32.7 ± 6.7 | 32.9 ± 6.5 | 0.86 |
| Second | 30.8 ± 6.1 | 31 ± 5.4 | 0.84 |
| Third | 28.1 ± 4.6 | 27.2 ± 5.2 | 0.32 |
| Forth | 30.6 ± 6.1 | 29.8 ± 3.8 | 0.34 |
| Fifth | 28.3 ± 5.1 | 27.9 ± 1.4 | 0.48 |
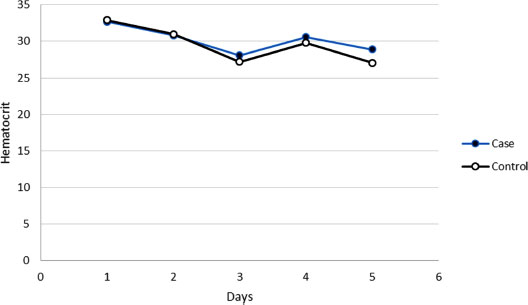
3.3. Hematocrit Levels
The hematocrit levels on different days were not significantly different between the groups (Table 3).
3.4. Hematocrit Trend
In groups of case and control, the hematocrit decreased for consecutive 5 days after surgery, although these decreases were not significant (p>0.05) (Fig. 1).
3.5. Hemoglobin Levels
The mean hemoglobin levels on different days was not significantly different between the two groups (Table 4).
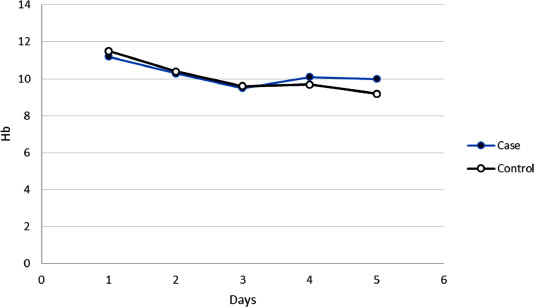
3.6. Hemoglobin Trend
In groups of case and control, the mean of hemoglobin decreased for consecutive 5 days after surgery, although these decreases were not significant (p>0.05) (Fig. 2).
Table 4.
| Day | Case group (n=47) | Control group (n=90) | P value |
| First | 11.2 ± 2.3 | 11.5 ± 2.2 | 0.45 |
| Second | 10.3 ± 2 | 10.4 ± 2.1 | 0.78 |
| Third | 9.5 ± 1.5 | 9.6 ± 2.3 | 0.79 |
| Forth | 10.1 ± 1.7 | 9.7 ± 2 | 0.24 |
| Fifth | 10 ± 1.6 | 9.8 ± 0.7 | 0.31 |
4. DISCUSSION
In this retrospective observational study, the medical files of 137 patients with GI cancer were included in two cases (ketorolac + pantoprazole) and control (ketorolac) groups. The mean age of the patients (range 18-60 years), the gender distribution (children and the elderly were excluded), and the comorbidities were not significantly different in the two groups.
The findings of the present study showed that in the patients of the case group who received pantoprazole as a prophylaxis regimen for stomach ulcers, GI complications (upper bleeding, melena, regurgitation, occult blood, ulcer bleeding) were reported in a smaller number of people, although compared to the control group, it was no significant difference.
In line with the present findings, Kim et al. reported a 36% reduction in the risk of occult GI bleeding in GI prophylaxis (NSAID) users compared with non-users. Further analysis revealed that PPIs, H2RAs, rebamipide and misoprostol significantly reduce the risk of GI damage [19]. Several investigations have shown that PPIs and H2RAs not only prevent upper GI tract damage in NSAID users but are also competing against large and small intestine damage [20, 21]. Therefore, in line with previous studies, our findings show that simultaneous GI prophylaxis with pantoprazole might be efficient in reducing GI damage in NSAID users. However, these findings were not significant.
PPIs and H2RAs are effective in preventing upper GI bleeding in NSAID users [22]. Currently, despite the prevalent use of PPIs, there are still some unresolved concerns about their potential risks. Some previous studies have indicated that the use of PPIs, through a possible mechanism of acid suppression in the stomach, may lead to an increased risk of several infectious diseases, including Clostridium difficile infection, other intestinal infections, pneumonia, and osteoporotic fractures [20, 23]. On the other hand, there are some concerns about possible medication interactions, which were not reported in the present study.
It is known that a decrease of 2 gr/dl of hemoglobin and/or a drop of hematocrit ≥ 10 is a clinical manifestation of upper or lower GI tract bleeding [24, 25] that can prompt physicians to clinical decisions or new orders. According to these points, in a number of comprehensive studies, this clinical outcome has been presented as an important outcome for GI tract evaluation in NSAID users. In some studies, it has been stated that compared to the examination of the evident events of the GI tract, the confirmation of hemoglobin reduction may be a better indicator of the effect of medications on the overall damage of the GI tract [26, 27]. In the current study, this clinical outcome was also considered, but the findings did not show a significant change in the amount of hemoglobin and hematocrit between the groups and also on different days.
In GI cancer surgery, NSAIDs such as ketorolac are used to control pain in patients [28]. Pain prevents the patient from daily activities and leads to long hours of absence from work. In the current study, although the ICU stay (hours) was not significantly different, the hospital stay (days) was significantly lower in the case group that received the GI prophylaxis regimen than in the control group that only received ketorolac. Early discharge from the hospital (shorter hospital stay) may be considered an indicator of proper pain relief and bleeding control.
Ketorolac is approved for post-surgical pain relief, but it has raised concerns about the potential serious side effects and even death. Among the side effects associated with ketorolac administration, platelet inhibition with changes in hemostasis, bleeding and perforation of the GI tract, and kidney failure can be mentioned. Since the revision of the dosage guidelines, the incidence of these serious side effects has decreased. Most previous studies have shown that the overall risk of GI bleeding or surgical site bleeding associated with ketorolac treatment is only slightly greater than with opioids. However, these side effects are more likely to manifest at high doses, long-term treatment (more than 5 days), or in vulnerable patients (such as the elderly). Acute renal failure has been reported after ketorolac therapy but is usually reversible after discontinuation. Like other NSAIDs, ketorolac may cause allergic or hypersensitivity reactions. Therefore, it is important to carefully select the patient when using ketorolac. Physicians should be familiar with and follow dosage warnings and instructions [28, 29].
Although GI bleeding after GI cancer surgery is rare, for example, in a cohort study in Canada in 2019, the incidence of intra-intestinal bleeding after GI surgery was reported to be 2.3% [30]. But the use of NSAIDs can aggravate GI bleeding after surgery, and if this bleeding is not properly controlled, it can be life-threatening. Therefore, a combination therapy that reduces the adverse effect of ketorolac on GI bleeding can be very important, so perhaps a suitable option is to use pantoprazole. Because in an experimental study in Egypt in 2015, it was shown that the simultaneous use of NSAIDs and PPIs combination is the best agent for the treatment and prevention of GI bleeding caused by the use of NSAIDs [14]. In the present study, this combination resulted in better outcomes. Although the mechanism of controlling GI bleeding by pantoprazole has not been fully clarified, it suppresses gastric acid secretion in gastric parietal cells by irreversibly inhibiting Hydrogen potassium ATPase [18].
Considering that no side effects were reported in the patients of the present study, it can be concluded that the administration of GI prophylaxis (pantoprazole) along with ketorolac is more effective in managing pain and unwanted complications including GI bleeding in patients who are candidates for GI cancer surgery, compared to Ketorolac alone. Of course, one thing that should not be forgotten is that the prescription of GI prophylaxis is dependent on the dose and type of PPIs, so it is very important to choose the right dose and type for maximum efficacy and minimum complications.
This study has some limitations. Firstly, a larger sample size with a prospective design will increase the validity of the data. On the other hand, although the aim of the present study was not to measure pain, and GI bleeding was considered the main outcome, pain measurement could also lead to a more precise interpretation of the findings, which was not done in the present study. Also, it would have been better if the unwanted side effects caused by the prescription of medications, such as the frequency of vomiting and nausea, as well as the patient's satisfaction and pain score (VAS), were recorded and reported at different time intervals in the short and long term. It is suggested that in future studies, in addition to GI bleeding, pain relief, side effects, and patients' satisfaction with analgesia should be evaluated and recorded in short and long-term intervals.
CONCLUSION
The current study showed that the use of a stomach ulcer prophylaxis regimen (pantoprazole) is effective and reduces the frequency of GI bleeding caused by ketorolac after GI cancer surgery, although this reduction was not significant. In line with previous studies and recent guidelines, the NSAID and PPIs combination may be considered one of the best agents for the treatment and prevention of GI bleeding damage caused by the use of NSAIDs in these patients.
LIST OF ABBREVIATIONS
| GI | = Gastrointestinal |
| NSAID | = Nonsteroidal Anti-inflammatory Drug |
| PPIs | = Proton Pump Inhibitors |
| H2RA | = Histamine-2 Receptor Antagonists |
| GCS | = Glasgow Coma Scale/Score |
| VAS | = Visual Analogue Scale |
| ICU | = Intensive Care Unit |
AUTHORS’ CONTRIBUTIONS
RH, and SZ were responsible for the study concept and design. RH, and SZ led data collection. MK, and MM were responsible for the analysis and interpretation of data. RH and SZ wrote the first draft. MK, and MM provided comments on initial drafts and coordinated the final draft. All authors read and approved the final manuscript. All authors take responsibility for the integrity of the data and the accuracy of the data analysis.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Institutional Review Board approval (Code: IR.MUMS.REC.1386.085).
HUMAN AND ANIMAL RIGHTS
No animals were used that are the basis of this study. All the human experiments were conducted in accordance with the Declaration of Helsinki Declaration.
CONSENT FOR PUBLICATION
Informed consent was obtained from all the participants.
AVAILABILITY OF DATA AND MATERIALS
The data used in this study are available from the corresponding author [M.M] upon request.
STANDARDS OF REPORTING
STROBE guidelines were followed in this study.
FUNDING
This study was funded by Rafsanjan University of Medical Sciences.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
Thanks to guidance and advice from the “Clinical Research Development Unit”. The authors gratefully acknowledge and, in memory of all medical staff, as well as thousands of unsung heroes who participate in the frontline in the fight against the epidemic of SARS-CoV-2.


