All published articles of this journal are available on ScienceDirect.
Hydrogen Peroxide Plasma Sterilization Sabotages the Efficacy of Lidocaine HCl Injection
Abstract
Background:
Lidocaine injection with 2% HCl as an anesthetic drug must guarantee its sterility to avoid microbial contamination. In efforts to maintain the sterile preparation of Lidocaine HCl 2% before use in hospitals, some anesthesiologists opt for re-sterilization.
Objective:
This study aimed to evaluate the impact of plasma sterilization using hydrogen peroxide on Lidocaine HCl levels employing a validated Ultra Performance Liquid Chromatography (UPLC) assay.
Methods:
The 2% Lidocaine HCl samples were separated into two groups, one undergoing re-sterilization with hydrogen peroxide and the other handled only with aseptic techniques. The chromatographic assay was performed using a Waters Corp Acquity UPLC® H-Class system and a Waters Corp Acquity UPLC® BEH C18 column, with a mobile phase of 20% Acetonitrile and 80% Acetate Buffer pH 3.4, flow rate of 0.3 mL/min, and total duration of 4.5 minutes.
Results:
The results showed a decrease in Lidocaine HCl levels to 1.88% after re-sterilization and 2.01% without re-sterilization.
Conclusion:
These findings suggest that re-sterilization with hydrogen peroxide plasma sterilization leads to a significant decrease in Lidocaine HCl levels, causing non-compliance with pharmacopoeia standards.
1. INTRODUCTION
Handling anesthesia injections requires strict adherence to aseptic techniques as they are in a sterile form. Sterile preparations are characterized by several unique features, including a lack of bacteria, pyrogens, and particles, as well as stringent requirements for purity and quality [1]. As far as injection preparations are handled aseptically, they will remain sterile until administered to the patient since their sterility is guaranteed.
When handling sterile preparations, health practitioners must be mindful of contamination as well as the safety of people, products, and the environment [2]. The work practices that minimize microbial contamination and lower the danger of worker exposure are known as aseptic techniques. The ultimate goal of producing and handling sterile preparations is to eliminate all instances of microbiological contamination [3].
However, an anesthesiologist occasionally requests for anesthesia injection to be sterilized once more before being administered to patients. There have not been any reports of the incidence of infections brought on by re-sterilized lidocaine until today.
A local anesthetic lidocaine hydrochloride injection is used to temporarily numb a small area of the body prior to performing minor surgery, such as a skin biopsy [2]. A sterile solution of lidocaine hydrochloride in water for injection or a sterile solution formulated from lidocaine and hydrochloric acid P in water for injection serve as the preparation for injecting lidocaine. Pharmacopoeia stated the requirement of Lidocaine hydrochloride, C14H22N2O. HCl should be kept in a concentration of neither less than 95.0% nor more than 105.0% of the amount stated on the label [4].
Re-sterilizing lidocaine often involves either using an autoclave or hydrogen peroxide plasma gas sterilization. Plasma sterilization is one of the sterilizers that can be applied during the re-sterilization procedure. Microorganisms are mostly rendered inert by hydrogen peroxide plasma gas sterilization, which also produces free radicals during the plasma phase of the cycle [5]. The use of hydrogen peroxide plasma gas sterilization has the benefit of being safe for patients, medical personnel, and the environment because the sterilization procedure is done at low temperatures. However, there are a number of downsides to hydrogen peroxide plasma gas sterilization, including expensive prices, the fact that it is ineffective when there is moisture, cellulose, or cotton present, and poor penetration because of condensation [4].
Anesthesiologists in hospitals insist on the re-sterilization of 2% lidocaine in injection preparations to adhere to microbiological standards that limit the endotoxin content to 1.1 units per mg of preparation [6]. However, the choice of re-sterilization method is critical as it can potentially affect the potency and anesthetic effectiveness of lidocaine. In a study by Bridenbaugh and Moore (1958), it was demonstrated that Lidocaine (Xylocaine) 1% and 2% solutions could be autoclaved at 255 to 260°F under 18 to 20 lb. of pressure for 30 minutes without a significant loss of potency that would alter the expected clinical outcomes [7].
Although autoclaving could maintain lidocaine levels and clinical outcomes, its impact on anesthetic effectiveness needs to be considered. Additionally, patient variables, such as resistance to lidocaine or concomitant medication usage, can also influence the anesthetic effect [8]. Therefore, pharmacists play a crucial role in ensuring the quality of lidocaine preparation, even in cases of re-sterilization. Considering the limitations of high-temperature sterilization methods, some hospitals have explored alternative approaches, such as low-temperature sterilization using hydrogen peroxide plasma gas. This study aimed to evaluate the impact of re-sterilization with hydrogen peroxide plasma gas on the concentration of 2% lidocaine HCl to indirectly assess its anesthetic efficacy.
2. MATERIALS AND METHODS
This study is an experimental research involving fourteen ampoules of Lidocaine HCl 2% injection produced by PT. Phapros Tbk., Semarang, Indonesia. Seven ampoules were re-sterilized using hydrogen peroxide plasma gas and the other seven were not re-sterilized. The equipment used in this study was the low-temperature sterilization machine SterradTM 100S Sterilizer manufactured by Advance Sterilization Products (ASP Global Manufacturing, GmbH, Irvine, CA, USA). Both ampoules were aseptically handled before assaying using the Ultra Performance Liquid Chromatography (UPLC) method. Lidocaine HCl BPFI (purchased from the National Food and Drug Administration-BPOM, Jakarta Indonesia) was used as reference standard. Acetonitrile Gradient Grade for Liquid Chromatography LiChrosolv Reag. Ph Eur (Merck, Darmastadt, Germany) and water pro-HPLC were obtained from PT. Ikapharmindo Putramas, Jakarta, Indonesia.
The chromatographic assay was performed on an Acquity UPLC® H-Class system (Waters Corp., Milford, MA, USA) equipped with a quaternary solvent manager, sample manager, a column heater, and a UV-visible detector. The separation was carried out on an Acquity UPLC® BEH C18 column (50 × 2.1 mm i.d., 1.7 μm Waters Corp., Wexford, Ireland). An injection volume of 1.40 μL was used, and the isocratic elution was performed using a mobile phase of 20% Acetonitrile and 80% Acetate Buffer pH 3.4 in Aqua Pro HPLC, with a flow rate of 0.3 ml/min for a total duration of 4.5 minutes. Maximum wavelength was determined using a UV spectrophotometer (UV-1800 Shimadzu, Shimadzu Corp, Kyoto, Japan).
The concentration of lidocaine HCl in the injection solution was measured using the modified procedure from the Indonesian Pharmacopoeia Sixth Edition, which involved using High-Performance Liquid Chromatography (HPLC) with UPLC to reduce the injection volume and observation time. Before conducting the sample measurements, the analytical method was validated by conducting tests for system suitability, specificity, limit of quantification (LOQ), limit of detection (LOD), linearity, accuracy, and precision.
About 52.06 mg of Lidocaine HCl was accurately weighed using a certified analytical balance. The standard was then dissolved in 10 mL acetate buffer with a pH of 3.4 to create a stock solution with a concentration of 5206 ppm. This stock solution was diluted with the same solvent to produce a range of standard solutions at concentrations of 10.41, 20.82, 41.65, 83.30, 166.59, 208.24, 249.89, and 312.36 ppm. Before undergoing UPLC analysis, the samples were filtered through a 0.2 µm filter.
The process of sterilizing Lidocaine HCl using hydrogen peroxide plasma gas sterilization was carried out at Hospital X. In this study, fourteen samples (ampoules) of Lidocaine HCl 2% were divided into 2 groups. The first group was re-sterilized using hydrogen peroxide plasma sterilization, and the second group was only handled using aseptic techniques. The sterilization process was carried out as set in Table 1. A total of 7 ampoules were put into the SterradTM 100s sterilizer for the sterilization process.
Prior to the analysis, it was essential to validate the Ultra Performance Liquid Chromatography (UPLC) assay method used for quantification to ensure accurate and reliable measurements. Therefore, the assay method was rigorously validated, considering parameters, such as linearity, specificity, accuracy, precision, limit of quantification (LOQ), and limit of detection (LOD).
| Phase | Duration | Pressure |
|---|---|---|
| Vacuum stage | 19 min 58 sec | 397 torr |
| Injection stage | 6 min 2 sec | 9,23 torr |
| Diffusion stage | 2 min 0 sec | 15 torr |
| Plasma stage | 6 min 54 sec | 495 torr |
| Injection stage | 6 min 1 sec | 9,77 torr |
| Diffusion stage | 2 min 1 sec | 15 torr |
| Plasma stage | 6 min 45 sec | 502 torr |
| Total sterilization time | 49 min 41 sec | |
- daily cycle #1
- total machine cycles 75
- short cycle.
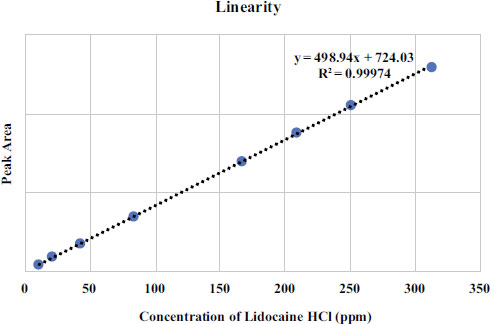
The linearity of the lidocaine HCl BPFI calibration curve was assessed over a concentration range of 10.41 to 312.36 μg/mL. Each point on the calibration curve was tested in triplicate for validation. The average peak area was determined and plotted against the sample concentration to generate the curve. Three calibration curves were created on the same day. The standard curve equation was obtained by performing a least squares linear regression analysis from the linearity curve of Lidocaine HCl as shown in Fig. (1) The linearity results indicate that there is a direct correlation between the concentration and the peak area of Lidocaine HCl standard solution with the correlation coefficient of r 2 ≥ 0,9997 in the measurement concentration ranging from 10.41 μg/mL to 312.36 μg/mL. The validation results are summarized in Table 2.
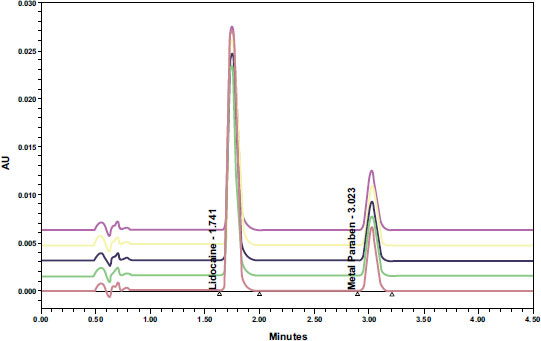
To determine the specificity of the analytical method, 1.4 µl solvent, a standard solution of pure lidocaine HCl BPFI (analyte), and methylparaben as the interferent component were injected into the system with a run time of 2.5 minutes. Fig. (2) reveals that the UPLC method detects the presence of interferences and their retention time, with Lidocaine HCl having a retention time of 1.745 minutes, methyl paraben at 3.020 minutes, and solvents not showing any peaks at the Lidocaine HCl's retention time. The chromatograms show that the two peaks, Lidocaine HCl and methylparaben, are well separated and meet the requirement of having a minimum RS value of 2.5. Based on the system conformity test results in Table 3, the peak area %RSD of Lidocaine HCl was found to be 0.19%, which satisfies the requirement of being ≤ 2%. Similarly, the retention time %RSD of Lidocaine HCl was 0.20%, meeting the requirement of being ≤ 2%. The resolution was determined to be 8.84, exceeding the requirement of being >1.5. The plate count was 2825.04, which meets the requirement of being >2000, and the Tailings Factor was 1.51, meeting the requirement of being ≤ 2.
| Validation Parameter | Results for Lidocaine HCl | Reference |
|---|---|---|
| Linearity (r2) | 0.99974 | ≥ 0.98 |
| Linearity rangea | 10.41 to 312.36 μg/mL | N.A |
| Accuracy (%) | 99.89 to 100.96% | 95 to 105% |
| Precision (%RSD) | 0.68% | %RSD Area ≤ 2% |
| Resolution | 8.84 ± 0.09 (%RSD 1.0) | ≤ 2 |
| Retention time | 1.745 minutes (%RSD 0,20) | %RSD Rt ≤ 2% |
| Plate count | 2825.04 ± 39.07 | > 2000 |
| Tailing Factor | 1.51 ± 0.01 | ≤ 2 |
| LOD | 6.00 μg/mL | N.A |
| LOQ | 20.01 μg/mL | N.A |
| Repeat | Lidocaine | Methyl Paraben | Resolution | Lidocaine | Methyl Paraben | ||||
|---|---|---|---|---|---|---|---|---|---|
| Area | Rt | Area | Rt | Plate Count | Tailing Fac. | Plate Count | Tailing Fac. | ||
| 1 2 3 4 5 6 7 8 9 10 |
110563 110620 110488 110919 110517 110691 110460 110883 110487 111054 |
1.741 1.742 1.74 1.749 1.744 1.748 1.745 1.748 1.745 1.75 |
34773 34727 34768 34947 34629 34744 34809 34716 34764 34784 |
3.023 3.022 3.018 3.028 3.02 3.022 3.018 3.020 3.016 3.017 |
9.01 8.93 8.87 8.85 8.83 8.82 8.77 8.83 8.81 8.68 |
2903.01 2870.94 2828.57 2832.97 2804.55 2825.78 2795.52 2803.13 2821.61 2764.28 |
1.51 1.50 1.51 1.52 1.53 1.52 1.51 1.50 1.50 1.50 |
6355.85 6278.24 6215.52 6279.49 6306.38 6213.82 6218 6229.03 6272.37 6080.08 |
1.14 1.15 1.16 1.16 1.17 1.16 1.16 1.16 1.16 1.17 |
| Mean | 110668.20 | 1.745 | 34766.10 | 3.020 | 8.84 | 2825.04 | 1.51 | 6244.88 | 1.16 |
| SD | 211.69 | 0.20 | 80.46 | 0.004 | 0.09 | 39.07 | 0.01 | 73.88 | 0.01 |
| %RSD | 0.19 | 0.20 | 0.23 | 0.12 | 1.00 | 1.38 | 0.70 | 1.18 | 0.76 |
Accuracy and precision were determined by measuring the peak areas of chromatograms of three standard solution concentrations of 166.59, 208.24, and 249.89 μg/mL in triplicates. The results were expressed as a percentage of relative standard deviation (%RSD) in the measurement. The results showed that the accuracy and precision met the required standards (%recovery of 99.0% to 100.96%), with a %recovery of 100.96% and precision (%RSD ≤ 2%) of 0.68%. The quantification limits and detection limits of Lidocaine HCl in solution were determined by injecting standard solutions at concentrations of 10.41 μg/mL to 312.36 μg/mL and creating a calibration curve between concentration and peak area. The regression line equation was calculated as y = 498.94x + 724.02. The limit of quantification was 20.01 μg/mL, and the limit of detection was 6.00 μg/mL.
3. RESULTS
The concentration of Lidocaine HCl, 2% injection, was determined by testing two groups of seven ampoules from three batches of the solution. Within each ampoule, triplicate measurements were performed to ensure accuracy. The mean value of the three measurements for each ampoule was then considered as the representative measurement for the concentration of Lidocaine HCl in that specific ampoule. The UPLC Determination results of Lidocaine HCl without and with re-sterilization are provided in Tables 4 and 5.
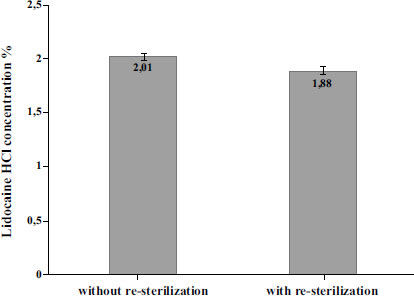
The results, as summarized in Fig. (3), revealed that the concentration of Lidocaine HCl in the 2% injection without re-sterilization was 2.0%. This concentration falls within the quality requirements specified by the Indonesian Pharmacopoeia (95.0%-105.0) and aligns with the information provided on the drug label. However, the concentration of Lidocaine HCl was found to decrease to 1.88% after undergoing re-sterilization with hydrogen peroxide plasma sterilization. The statistical analysis was performed using a confidence level of 0.1%. This level of significance was chosen to assess the statistical significance of the results. As the data did not follow a normal distribution, non-parametric analysis was performed using the Mann-Whitney test. The difference in Lidocaine HCl concentration without and with sterilization was analyzed statistically using the Mann-Whitney non-parametric test, which showed a significant difference (p-value of 0.001) between the two groups. Results showed that the lidocaine HCl concentrations in the ampoules without re-sterilization had a mean concentration of 2.01% (SD 0.03%, RSD 1.54%). After re-sterilization, the lidocaine HCl concentrations were found to have a mean concentration of 1.88% (SD 0.0038%, RSD 0.20%). The small standard deviation resulting in a narrow confidence interval indicated a high level of precision and consistency in the measurements.
| No. | Sample Name | Volume (mL) | Dilution until (mL) |
Peak Area (Measured in Triplicate) |
Average Peak Area | Factual Concentration (µg/L) | Dilution Factor | Lidocaine Concentration (µg/L) | Lidocaine Level (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||||||
| 1 | 1 – A | 0,04 | 5 | 80166 | 80052 | 80079 | 80099,00 | 159,09 | 125 | 19885,83 | 1,99 |
| 2 | 1 – B | 0,05 | 5 | 100665 | 100768 | 100749 | 100727,33 | 200,43 | 100 | 20043,08 | 2,00 |
| 3 | 1 – C | 0,06 | 5 | 120326 | 120371 | 120217 | 120304,67 | 239,67 | 83 | 19972,38 | 2,00 |
| 4 | 2 – A | 0,05 | 5 | 100665 | 100768 | 100749 | 100727,33 | 200,43 | 100 | 20043,08 | 2,00 |
| 5 | 2 – B | 0,05 | 5 | 103414 | 103545 | 103178 | 103379,00 | 205,75 | 100 | 20574,54 | 2,06 |
| 6 | 3 – A | 0,05 | 5 | 99048 | 99091 | 99316 | 99151,67 | 197,27 | 100 | 19727,28 | 1,97 |
| 7 | 3 – B | 0,05 | 5 | 102451 | 102320 | 102376 | 102382,33 | 203,75 | 100 | 20374,78 | 2,04 |
| - | - | - | - | - | - | - | - | - | - | Mean | 2,01 |
| - | - | - | - | - | - | - | - | - | - | SD | 0,03 |
| - | - | - | - | - | - | - | - | - | - | %RSD | 1,54 |
| No. | Sample Name | Volume (mL) | Dilution until (mL) |
Peak Area (Measured in Triplicate) |
Average Peak Area | Factual Concentration (µg/L) | Dilution Factor | Lidocaine Concentration (µg/L) | Lidocaine Level (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||||||
| 1 | 1 – D | 0,04 | 5 | 75916 | 75822 | 75990 | 75909,33 | 150,69 | 125 | 18836,19 | 1,88 |
| 2 | 1 – E | 0,05 | 5 | 94466 | 94706 | 94495 | 94555,67 | 188,06 | 100 | 18806,13 | 1,88 |
| 3 | 1 – F | 0,06 | 5 | 113518 | 113585 | 113580 | 113561,00 | 226,15 | 83 | 18846,05 | 1,88 |
| 4 | 2 – D | 0,05 | 5 | 94466 | 94706 | 94495 | 94555,67 | 188,06 | 100 | 18806,13 | 1,88 |
| 5 | 2 – E | 0,05 | 5 | 94903 | 95257 | 94666 | 94942,00 | 188,84 | 100 | 18883,56 | 1,89 |
| 6 | 3 – D | 0,05 | 5 | 94758 | 94437 | 94738 | 94644,33 | 188,24 | 100 | 18823,90 | 1,88 |
| 7 | 3 – E | 0.05 | 5 | 94401 | 94222 | 94584 | 94402,33 | 187,75 | 100 | 18775,40 | 1,88 |
| - | - | - | - | - | - | - | - | - | - | Mean | 1,88 |
| - | - | - | - | - | - | - | - | - | - | SD | 0,0038 |
| - | - | - | - | - | - | - | - | - | - | %RSD | 0,20 |
4. DISCUSSION
The results of the study provide insights into the impact of hydrogen peroxide plasma sterilization on the efficacy of Lidocaine HCl injection. Lidocaine HCl, a commonly used anesthetic drug, must maintain its sterility to prevent microbial contamination and ensure patient safety.
The aim of this study was to examine the effect of re-sterilizing Lidocaine HCl 2% anesthetic drug using hydrogen peroxide plasma on its concentration. Maintaining the sterility of Lidocaine HCl 2% is crucial to prevent contamination by microbes. Some anesthesiologists choose to re-sterilize the preparation to ensure its sterility, but this may alter the concentration of the drug and impact its effectiveness.
The study used Ultra Performance Liquid Chromatography (UPLC) to assess changes in the concentration of Lidocaine HCl 2% after re-sterilization. In this study, two groups of Lidocaine HCl 2% samples were tested: one group was re-sterilized using hydrogen peroxide plasma, and the other was treated only using aseptic techniques.
Lidocaine HCl, commonly used in pharmaceutical formulations, such as creams and injections, is typically subjected to sterilization processes to ensure the preservation of the active ingredients. Traditional high-temperature sterilization methods are known to potentially cause damage to the medication [9]. As an alternative to mitigate such risks, low-temperature sterilization techniques, such as hydrogen peroxide plasma gas sterilization, have been explored. Hydrogen peroxide acts as an oxidizing agent, exerting its sterilization effect through the oxidation of vital cellular components. On the other hand, gas plasmas, which are highly ionized gases comprising ions, electrons, and neutral particles, provide a distinct state of matter with a visible glow. Gas plasmas demonstrate various mechanisms of action for sterilization, including the generation of reactive species that can harm cellular components and the production of UV radiation capable of causing DNA damage in microorganisms [10].
In this study, after the re-sterilization process with hydrogen peroxide plasma sterilization, the concentration of the active ingredient in the 2% Lidocaine HCl preparation was measured using Ultra Performance Liquid Chromatography (UPLC). Advantages of UPLC include faster analysis time, increased sensitivity and selectivity, maintaining resolution performance, and reducing operating costs [11].
The maximum wavelength for Lidocaine HCl was determined using a UV-Vis spectrophotometer instrument (UV-1800 Shimadzu) and was found to be 271 nm. The chromatographic separation was achieved in 14 minutes, using a gradient elution method with an Eclipse Agilent plus C18 (100x4.6) mm, 1.8μm column, and a buffer solution of potassium dihydrogen phosphate (pH 4.50) mixed with acetonitrile. The eluted compounds were detected at 230 nm with a photodiode array (PDA) detector at a flow rate of 1.0 mL/min, with the column oven temperature kept at 40°C. The difference in the wavelengths obtained in this study compared with previous studies can be explained by the solvent used and the equipment specifications [12]. The maximum wavelength of Lidocaine HCl can be measured using a UV-Vis spectrophotometer due to its chromophore groups [13].
The concentration of the Lidocaine HCl preparation was determined using the UPLC instrument and the ACQUITY UPLC BEH C18 column, which is considered the ideal column for most UPLC separations. The UPLC analysis showed a peak for Lidocaine at a retention time of 1.745 minutes and a peak for methyl paraben at a retention time of 3.020 minutes. The separation of the two peaks was considered adequate based on the RS values being ≥2.5. The specificity of the test [6].
Ultra-Performance Liquid Chromatography (UPLC) has emerged as a more efficient and cost-effective alternative to High-Performance Liquid Chromatography (HPLC) for analyzing Lidocaine HCl in an aqueous injection. UPLC allows for smaller injection volumes, shorter run times of less than 5 minutes, and reduced solvent usage. Furthermore, UPLC provides improved peak resolution due to the use of columns and higher operating pressures [6].
The analytical method used in this study was thoroughly validated to ensure the quality of the assay. Linearity testing resulted in a correlation coefficient (r) value of 0.9997, indicating good linearity (40). The limit of detection (LOD) value was found to be 6.00 µg/mL, representing the lowest detectable concentration of lidocaine using the UPLC instrument. The limit of quantification (LOQ) value was measured as 20.01 µg/mL, indicating the lowest concentration of lidocaine that meets accuracy and precision criteria [6]. Precision and accuracy testing demonstrated that the analytical method produced good recovery values, with %RSD < 2% and % recovery values for Lidocaine HCl samples in the range of 97.2-102.9% [14].
The sample size was chosen based on ICH guidelines, which recommend testing a minimum of three batches of the drug substance or product to establish stability information. In this study, seven samples from three different batch numbers were used, which is consistent with the ICH guidelines [15]. The study demonstrated high precision as evidenced by the low relative standard deviation %RSD (%RSD Area ≤ 2%) obtained from all replicates during method validation and system conformity test. The concentration of Lidocaine HCl was determined on both samples without and with re-sterilization using hydrogen peroxide plasma. The initial concentration of 2.0% Lidocaine HCl met the standards specified on the drug label. However, after re-sterilization, the concentration dropped to 1.88%. To assess the impact of re-sterilization using hydrogen peroxide plasma on Lidocaine HCl concentration, we conducted a Mann-Whitney non-parametric test. The test revealed a statistically significant difference (p-value of 0.034) between the two groups (unsterilized and re-sterilized), indicating that the concentration of Lidocaine HCl is indeed affected by the re-sterilization process using hydrogen peroxide plasma.
There has been no previous research regarding the re-sterilization of Lidocaine HCl using hydrogen peroxide plasma. However, similar studies on autoclave sterilization at 121ºC for 15 minutes showed a decrease in Lidocaine HCl levels, although not significant [16]. The reduction in Lidocaine HCl concentration after re-sterilization could result from oxidative degradation. This notion is supported by prior research, where it was found that 24 hours of exposure of lidocaine to hydrogen peroxide (H2O2) led to a 7.25% decrease in lidocaine levels [17]. Although the hydrogen peroxide plasma sterilization was conducted for a short duration and there was no immediate interaction between Lidocaine HCl and hydrogen peroxide in this process, the observed decrease in Lidocaine HCl levels after re-sterilization suggests the occurrence of oxidative degradation.
The decrease in Lidocaine HCl levels after re-sterilization may have been caused by oxidative degradation, as previous research has shown that lidocaine can be reduced by 7.25% when dissolved with hydrogen peroxide for 24 hours [17]. This is in contrast to prior research that found Lidocaine HCl levels remain stable after sterilization using an autoclave [18]. Hydrogen peroxide plasma sterilizers are commonly used to inactivate prions in heat-sensitive medical instruments [19]. While our study focused on the impact of re-sterilization with hydrogen peroxide plasma gas, it is worth noting the findings from Bridenbaugh and Moore (1958), which support the viability of autoclaving Lidocaine solutions at specific temperature and pressure conditions without significant loss of potency. These results highlight the potential differences in the effects of various sterilization methods on the potency of Lidocaine and emphasize the need for further investigation into the specific impacts of different sterilization techniques on pharmaceutical compounds [7].
The next step after identifying the reduced levels of Lidocaine HCl after re-sterilization is to compare them with the required standards set by the Indonesian National Compendial. The Indonesian Pharmacopoeia and USP-NF specify that Lidocaine HCl should have a concentration range of 95.0% to 105.0% (1.90% to 2.10% for 2% Lidocaine HCl) of the labeled amount of lidocaine hydrochloride. The results of the study indicated that the Lidocaine HCl concentration was 1.88% after re-sterilization, which did not meet the compendial standards.
While our study provides insights into the impact of re-sterilization on Lidocaine HCl concentration, there are limitations that should be acknowledged. The small sample size of seven ampoules in each group may limit the generalizability of our findings. Additionally, our study focused solely on the concentration of lidocaine HCl and did not investigate other factors that could affect its stability or potency. Furthermore, we did not assess the actual anesthetic efficacy of re-sterilized lidocaine HCl in clinical settings. Therefore, further studies are needed to evaluate the clinical implications and patient outcomes. Lastly, our study only examined re-sterilization using hydrogen peroxide plasma gas, and the generalizability to other sterilization methods is limited.
Caution is advised when using re-sterilized Lidocaine HCl due to the potential consequences of decreased depth and duration of anesthesia. Lower lidocaine concentrations may result in insufficient pain relief during medical interventions, leading to discomfort and increased sensitivity to pain for patients. This could potentially cause distress and anxiety during procedures. Additionally, inadequate anesthesia may affect the success of medical interventions, especially in surgical procedures, where patient movement or discomfort can hinder precision and delicate surgical work. Furthermore, maintaining the appropriate lidocaine concentration is essential for patient safety and optimizing procedural outcomes, as certain medical interventions require specific anesthesia levels to ensure patient well-being and satisfaction. Using re-sterilized Lidocaine HCl with lower concentrations may lead to unexpected pain or discomfort, potentially leading to complications, adverse reactions, and the need for additional doses or alternative anesthetic agents, introducing risks of drug interactions or side effects.
CONCLUSION
The research findings indicate that re-sterilization using hydrogen peroxide plasma significantly decreases the levels of Lidocaine HCl, falling below the standards set by the national compendial, which directly impacts its efficacy as an anesthetic. Caution is advised when using re-sterilized Lidocaine HCl, as lower concentrations may result in decreased depth and duration of anesthesia, potentially affecting its effectiveness in certain procedures or patient populations. While lower lidocaine concentrations may suffice for some interventions, they may prove inadequate for more invasive or complex surgeries. Therefore, clinicians must be vigilant and consider the potential limitations of re-sterilized Lidocaine HCl to ensure optimal anesthesia outcomes and patient safety during various medical interventions.
LIST OF ABBREVIATIONS
| UPLC | = Ultra Performance Liquid Chromatography |
| LOD | = Limit of detection |
| LOQ | = Limit of quantification |
| HPLC | = High-Performance Liquid Chromatography |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No humans or animals were used for the studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of our article are deposited in the University of Surabaya Repository and can be accessed using the following details: http://repository.ubaya.ac.id/id/eprint/44984 Reference number: 44984.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGEMENTS
Declared none.


